Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 13 - Issue 1 - 2026
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Ganesh Chandra Jagetia*, Kanan Ramthianghlima
Department of Zoology Mizoram University Tanhril, Aizawl-796 004 Mizoram, India.
*Corresponding author: Ganesh Chandra Jagetia, Department of Zoology Mizoram University Tanhril, Aizawl-796 004 Mizoram, India.
Received: June 11, 2021
Accepted: June 22, 2021
Published: July 01, 2021
Citation: Ganesh Chandra Jagetia, Kanan Ramthianghlima “Oltipraz (4-Methyl-5-Pyrazinyl-3h-1,2-Dithiole-3-Thione) Alleviates Doxorubicin-Induced Cardiotoxicity in Abino Rats”. Clinical Case Reports and Clinical Study, 4(4); DOI: 10.61148/2766-8614/JCCRCS/081
Copyright: © 2021 Ganesh Chandra Jagetia. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Doxorubicin is an anthracycline group of antibiotics that is frequently used in the treatment of various human neoplasias clinically. However, its adverse effect on the human heart is of major concern and limits its full clinical utilization. The present study was undertaken to alleviate the doxorubicin-induced myocardial toxicity by oltipraz an Nrf-2 inducer in albino rats. The albino rats were orally administered with 10 mg/kg body weight of oltipraz daily for three days before treatment with 15 mg/kg body weight of doxorubicin. The doxorubicin-induced myocardial stress indicated by an increase in the CK-MB activity, and lipid peroxidation accompanied by a reduction in the glutathione, glutathione-s-transferase, catalase and superoxide dismutase in the rat heart. The administration of 10 mg/kg oltipraz for three days before doxorubicin treatment reduced the CK-MB activity and lipid peroxidation significantly followed by the significant elevation in the activities of glutathione-s-transferase, catalase and superoxide dismutase and glutathione in the rat heart. Our study demonstrates that oltipraz an Nrf-2 inducer reduced doxorubicin-induced myocardial stress in the rats.
Introduction
Anthracyclines rank among the most effective anti-cancer drugs ever developed (Weiss, 1992). The first anthracyclines were isolated from the pigment-producing Streptomyces peucetius in the early 1960s and were named doxorubicin and daunorubicin (Arcamone et al., 1969). The anthracyclines (including doxorubicin, daunomycin, epirubicin, and idarubicin) are one of the most clinically useful groups of anticancer chemotherapeutics. These drugs are routinely employed in combination regimes with other groups of drugs in which each drug generally exhibits a different mechanism of action to increase tumor cell kill and to minimize chemotherapy induced resistance (Carter, 1975). Doxorubicin is clinically used in the treatment of breast cancer, bladder cancer, childhood solid tumors, lymphomas, myeloblastic leukemia, neuroblastoma, ovarian cancer, small cell lung cancer, soft tissue sarcomas and HIV-associated Kaposi’s sarcoma (Carvalho et al., 2009; Henriksen, 2018; Ishihara et al., 2019; Meredith and Dass, 2016; Minotti et al., 2004; Tam, 2013; Volkova and Russell, 2011).
The cancer patients undergoing doxorubicin therapy develop life-threatening myocardial toxicity after long-term survival and this limits the clinical application of doxorubicin (De Angelis et al., 2016)(Henriksen, 2018). The metabolism of doxorubicin leads to the formation of doxorubicin deoxyaglycone, doxorubicin hydroxyaglycone, doxorubicinol, doxorubicinol aglycone, and doxorubicin-semiquinone, which trigger cardiotoxicity in the patients undergoing doxorubicin therapy (Minotti et al., 1998, 2004; Licata et al., 2000, Kalyanaraman, 2020). The doxorubicin uses multiple mechanisms to induce cardiotoxicity however production of reactive oxygen species and subsequent lipid peroxidation, calcium dysregulation, and intervention in energy transfer lead to heart failure (Wenningmann et al., 2019). The doxorubicin produces free radicals in the cell mitochondria and their exacerbation in the presence of iron is the most widely accepted mechanism of doxorubicin-induced cardiomyopathies (Ichikawa et al., 2014; Luu et al., 2018). The ROS interact with mitochondrial DNA, proteins and lipid and disrupt the mitochondrial function of cardiomyocytes leading to toxic effects. In addition, scarcity of an antioxidant system in the heart also adds to the toxicity of doxorubicin (Agunbiade et al., 2019). Doxorubicin not only adversely affect the heart but it produces toxic effects on the brain, bone marrow, lung, liver, kidneys, and ovaries (Mazzotta et al., 2016; Tacar et al., 2013).
Materials and Methods
Chemicals
Doxorubicin (DOX) was procured from Biochem Pharmaceutical Industries, Mumbai, India. Ethylenediaminetetraacetic acid (EDTA), phenazine methosulphate (PMS), nitroblue tetrazolium (NBT), nicotinamide adenine dinucleotide reduced (NADH), glutathione reductase, thiobarbituric acid (TBA), trichloroacetic acid (TCA), 5,5-dithio2-nitrobenzoic acid (DTNB), glutathione reduced (GSH), 1-chloro,2,4-dinitrobenzene (CDNB), tert-butyl-hydroperoxide tetra ethoxy propane were purchased from Sigma Aldrich Chemical Co. Bangalore, India. Potassium dihydrogen phosphate, disodium hydrogen phosphate, hydrogen peroxide, dipotassium hydrogen phosphate and other routine chemicals were supplied by Merck India Limited, Mumbai, India. Oltipraz (OLT) was a kind gift from
Animal care and handling
Six to eight weeks old albino rats weighing 45-60 grams were procured locally and acclimatized to laboratory conditions before designing the experiment. The rats were cared for and handled following the recommendations of the World Health Organization, Geneva, Switzerland, INSA (Indian National Science Academy, New Delhi, India), and the NIH, USA Guide for the Care of Laboratory Animals, 2011. The animals were kept in sterilized polypropylene cages bedded with sterile paddy husk (procured locally) and had free access to standard rodent diet and water. The Animal Ethics Committee of the Mizoram University, Aizawl, India approved the study.
Preparation of oltipraz
The oltipraz is sparingly soluble in water therefore it was dissolved in 0.5% carboxymethylcellulose in sterile physiological saline (SPS), whereas doxorubicin was dissolved in sterile distilled water.
Experimental
The cardioprotective effect of oltipraz was studied by dividing the animals into the following groups according to the treatment:
Thirty hours after the administration of doxorubicin, the animals from all the groups were killed by cervical dislocation. The animals were dissected and blood was collected by cardiac puncture and allowed to stand on ice for 30 minutes and the serum was collected for the estimation of creatine kinase isoenzyme (CK-MB). Immediately after blood collection, the hearts were perfused with cold phosphate buffered saline (PBS) blot dried and homogenized in PBS for the estimation of antioxidants and lipid peroxidation (LOO).
CK-MB
The activity of CK-MB was measured in the serum of rats using a commercially available kit (Coral Clinical Systems, Goa, India) according to the manufacturer’s protocol. The absorbance of the samples was read at 340 nm using autoanalyzer.
Total proteins
The protein contents were determined using the modified method of Lowry.
Glutathione
The measurement of glutathione concentration was carried out by the modified method (Moron et al., 1979), where the proteins were precipitated by 25% TCA, and centrifuged and the supernatant was mixed with 0.2 M sodium phosphate buffer (pH 8.0) and 0.06 mM DTNB. The mixture was allowed to stand for 10 minutes at room temperature and the absorbance of the sample/s was read against the blank at 412 nm in a UV-VIS double beam spectrophotometer and the GSH concentration has been calculated from the standard curve.
Glutathione-S-transferase
Glutathione-S-transferase (GST) was determined by mixing the tissue homogenate with 0.1 M potassium phosphate buffer, 1 mM EDTA, glutathione reductase, 10 mM GSH, and 12 mM tert-butyl-hydroperoxide and left for 10 min at 37°C in a water bath (Habig et al., 1974). The absorbance was read against the blank at 340 nm using a double beam UV-VIS spectrophotometer.
Catalase
The catalase activity was assayed by the catalytic reduction of hydrogen peroxide as a measure of catalase activity as described earlier (Aebi, 1984) where hydrogen peroxide was added to the sample, mixed and incubated at 37°C in a water bath. The decomposition of hydrogen peroxide was monitored every 0, 5, 10 and 30 seconds by recording the absorbance against the blank at 240 nm using a UV-VIS double beam spectrophotometer.
Superoxide dismutase
The SOD activity was estimated using nitroblue tetrazolium (NBT) (Fried, 1975). The tissue homogenate sample was mixed with NBT, phenazine methosulphate and NADH. The reaction was stopped by adding acetic acid. The colour formed at the end of the reaction was extracted into n-butanol and measured at 560 nm against the blank using a UV-VIS double beam spectrophotometer.
Lipid peroxidation (LOO)
The lipid peroxidation was estimated according to the modified method (Gelvan and Saltman, 1990) where the tissue homogenate was mixed with trichloroacetic acid (15%), thiobarbituric acid (0.375%), and butylated hydroxytoluene (0.01%) in 0.25 N HCl and the mixture was incubated at 95°C for 25 min. The mixture was brought to room temperature, centrifuged at 8,000 g, the supernatant collected and the absorbance was recorded at 535 nm against the blank using a UV-VIS double beam spectrophotometer. The lipid peroxidation has been determined against a standard curve prepared with tetraethoxypropane. For all biochemical estimations, duplicate samples were used from each animal for various estimations listed above and a minimum of five animals was used for each concurrent group.
The significance between the treatments was determined using the Student’s ‘t’ test and one-way ANOVA with the application of Tukey’s posthoc test for multiple comparisons. A p value of <0.05 was considered statistically significant. Origin Pro 8.5 (Origin Lab Corporation, Northampton, MA, USA) was used for statistical analyses.
results
The results are represented as mean ±standard error of the mean in Table 1 and Figure 1-6.
|
Treatment
|
Mean± Standard error of the mean |
||||||
|
CK-MB (IU/mg protein) |
Glutathione (nmol/mg protein) |
Glutathione-S-transferase (nmol/mg protein) |
Catalase (U/mg protein) |
Superoxide dismutase (nmol/mg protein) |
Lipid peroxidation (nmol/mg protein) |
|
|
|
Sterile Saline |
39.61±3.66 |
20.85±0.25 |
21.55±2.35 |
0.25±0.02 |
13.62±1.23 |
2.33±0.33 |
|
|
Oltipraz |
28.30±1.67 |
19.00±0.5 |
19.00±2.121 |
0.24±0.038 |
14.27±1.61 |
2.75±0.05 |
|
|
Doxorubicin |
102.17±5.90* |
3.43±0.57* |
2.96±0.05* |
0.10±0.001* |
2.059±0.84* |
11.33±1.66* |
|
|
Oltipraz + Doxorubicin |
67.93±5.92# |
16.33±0.68# |
16.33±0.68# |
0.20±0.010@ |
12.96±2.13# |
3.933±0.3# |
|
*p< 0.001 When compared to control. #p< 0.001, @p <0.05 When compared to doxorubicin alone treatment. N=5.
Table 1: Alteration in the biochemical profile of albino rat serum/heart treated with oltipraz for three consecutive days before 15 mg/kg body weight doxorubicin administration.
Creatinine Kinase-Mb
The creatinine kinase is an indicator of cardiotoxicity. The CK-MB activity in the non-drug treated rat heart was 39.61±3.66 IU. Oltipraz alone treatment non-significantly reduced the CK-MB activity in the serum to 28.30±1.67 IU (Table 1). Doxorubicin alone treatment significantly increased CK-MB activity (102.18±5.90 IU) when compared to spontaneous level by 2.6 folds (Table 1, Figure 1). Oltipraz administration before doxorubicin treatment significantly reduced the CK-MB activity (67.93±5.92 IU), by 1.5 folds (Table 1, Figure 1).
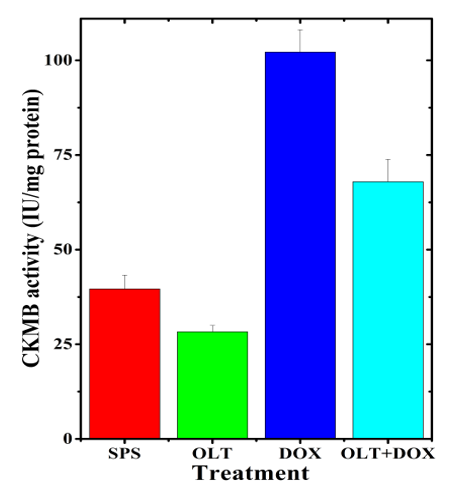
Figure 1: Alteration in the doxorubicin-induced CK-MB activity in the rat serum by oltipraz.
Glutathione
The level of glutathione in the untreated heart of rats was 20.85±0.25 nmol/mg protein. Oltipraz alone treatment did not significantly alter the GSH concentration when compared to non-drug treated control (Table 1). Doxorubicin alone treatment resulted in a significant decrease in the glutathione (3.43±0.57 nmol) concentration, which was 6 fold lower than the untreated control (Table 1, Figure 2). Oltipraz treatment before doxorubicin administration caused a significant rise in the glutathione concentration when compared to doxorubicin treatment alone (16.33±0.68 nmol), which was 4.8 fold higher than the latter (Table 1, Figure 2).
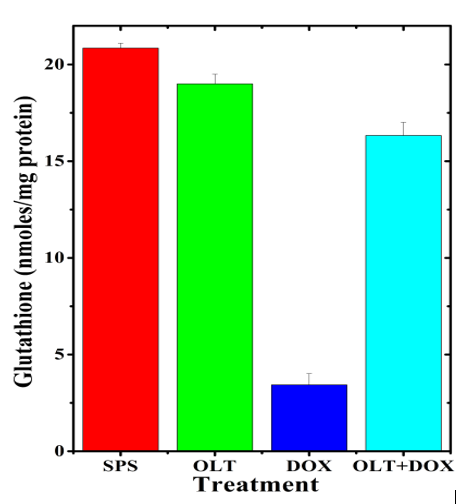
Figure 2: Alteration in the doxorubicin-induced glutathione concentration in the rat heart by oltipraz.
Glutathione-S-Transferase
The activity of glutathione-s-transferase (GST) in the untreated rat heart was 21.55±2.35 nmol/mg protein. Oltipraz alone treatment did not show significant changes in the GST activity as compared to control (Table 1). Administration of doxorubicin resulted in a drastic but significant reduction in the GST activity, which reduced to 2.964±0.045 (Table 1 and Figure 3). Treatment of rats with oltipraz before doxorubicin administration resulted in a significant elevation in the glutathione-S-transferase activity (16.33±0.676 nmol), which was 5.5 fold higher when compared to doxorubicin treatment alone (Table 1, Figure 3).

Figure 3: Alteration in the doxorubicin-induced glutathione-s-transferase activity in the rat heart by oltipraz.
Catalase
The catalase activity of the untreated rat heart was 0.25±0.02 U/mg protein. The oltipraz alone treatment did not alter the catalase activity significantly when compared to the spontaneous activity (Table1). Doxorubicin alone treatment significantly reduced the catalase activity (0.10±0.001) as compared to non-drug treated controls (Table 1, Figure 4). Oltipraz treatment elevated the catalase activity significantly when compared to doxorubicin treatment alone (Table 1, Figure 4). Administration of oltipraz before doxorubicin treatment led to a 2 fold rise in the catalase activity (Table 1).
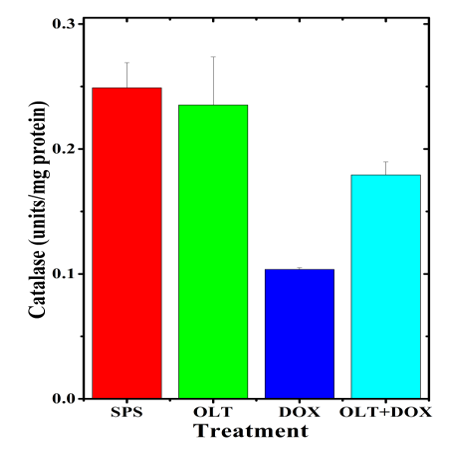
Figure 4: Alteration in the doxorubicin-induced catalase activity in the rat heart by oltipraz.
Superoxide Dismutase
The activity of SOD in the non-drug treated hearts of rat was 13.62±1.23 nmol/mg protein and treatment of rats with oltipraz alone did not significantly (14.27±1.60 nmol) change this activity(Table 1). Doxorubicin alone treatment also showed a significant decrease in the SOD activity (2.06±0.84 nmol), which was almost 6.3 fold lower than that of non-drug treated control (Table 1, Figure 5). Oltipraz treatment before doxorubicin administration elevated the SOD activity, which reached to almost normal level (12.96±2.13 nmol) in the oltipraz+doxorubicin treated group (Table 1, Figure 5).
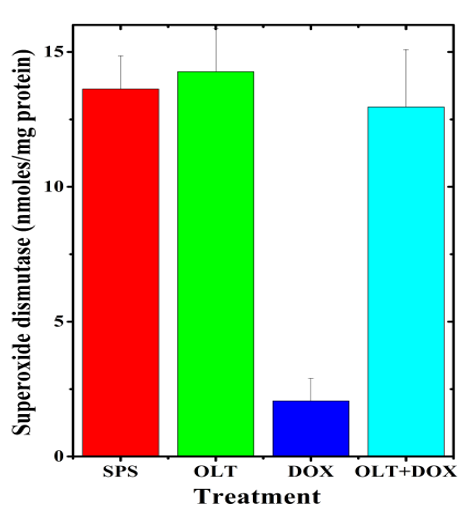
Figure 5: Alteration in the doxorubicin-induced superoxide dismutase activity in the rat heart by oltipraz.
Lipid Peroxidation
The results of the present study showed that the rate of lipid peroxidation in the untreated rat heart is 2.33±0.33 nmol/mg protein. Oltipraz alone treatment did not show significant alteration in the lipid peroxidation when compared to control (Table1, Figure 6). The doxorubicin alone treatment raised the level of lipid peroxidation (11.33±1.66 nmol) significantly when compared to the untreated control. This rise in lipid peroxidation after doxorubicin treatment was 4.9 fold higher when compared to non-drug treated control (Table 1). Administration of oltipraz before doxorubicin treatment significantly reduced lipid peroxidation (3.93±0.29 nmol), which was 2.9 fold lower as compared to doxorubicin treatment alone (Table 1, Figure 6).
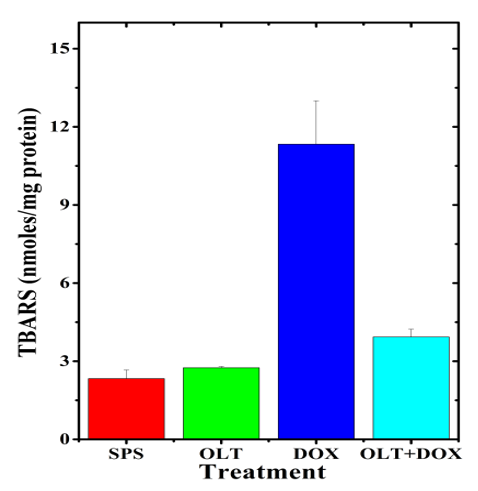
Figure 6: Alteration in the doxorubicin-induced lipid peroxidation in the rat heart by oltipraz.
Discussion
Cardiotoxicity is an important health concern for patients receiving doxorubicin for the treatment of cancer because it is expressed after many years of doxorubicin administration and remains a life-long threat. In recent years, several mechanisms have been suggested for doxorubicin-induced cardiotoxicity, however, the free radical theory is still the most accepted mechanism. Doxorubicin binds to cardiolipin present abundantly in the mitochondrial membrane leading to its accumulation in the mitochondria of the cardiomyocytes (Gorini et al., 2018). The quinone moiety of doxorubicin undergoes univalent reduction into a semiquinone radical, which is autooxidized in the presence of molecular oxygen to re-form the parent quinone radical and the superoxide anion as a free radical. This is a repetitive cyclic process that continually forms superoxide anions. The paucity of enzymes that passivate free radicals in the heart is the main cause of doxorubicin-induced cardiotoxicity (Agunbiade et al., 2019). The redox cycling of quinone moiety is catalyzed by the enzyme flavin dehydrogenase and other proteins including complex 1 of the electron transport chain located in the mitochondria and NADPH oxidase-2 present in the cardiac membrane (Doroshow et al., 2020). The doxorubicin interacts with iron to form doxorubicin iron complexes resulting in the cycling of iron between Fe3+ and Fe2+ increasing ROS production including the hydroxyl radical a highly toxic species via Fenton and Haber-Weiss reactions triggering toxic effects (Gammella et al., 2014).
The administration of doxorubicin-induced cardiotoxicity in the rats indicated by an increase in the CK-MB activity by 2.6 folds. The elevated serum levels of CK-MB have been considered as a reliable marker of doxorubicin-induced cardiotoxicity (Kemp et al., 2004). The doxorubicin has been reported to increase the activity of CK-MB in mice, rats, and rabbits earlier (Abdel-Raheem and Abdel-Ghany, 2009; Ahmed et al., 2021; Ajmal et al., 2015; Baniahmad et al., 2020; Jagetia et al., 2005; Jagetia and Reddy, 2014; Jagetia and Venkatesh, 2015; Warpe et al., 2015; Xiong et al., 2018; Zilinyi et al., 2018) The administration of rats with oltipraz reduced serum levels of CK.MB indicating that it protected against the cardiotoxic effect of doxorubicin. Oltipraz has been reported to protect rats against isoproterenol-induced heart failure (Tang et al., 2018). Earlier a polyherbal preparation, antarth, Agele marmelos (bael), naringin, hesperidin, ellagic acid, carvedilol metformin, berberine, vanillic acid and chia seed oil have been reported to protect mice, rats and rabbits against the doxorubicin-induced cardiotoxicity(Jagetia et al., 2005)(Jagetia and Reddy, 2014)(Abdel-Raheem and Abdel-Ghany, 2009) (Ajmal et al., 2015)(Warpe et al., 2015)(Jagetia and Venkatesh, 2015) (Zilinyi et al., 2018)(Xiong et al., 2018) (Baniahmad et al., 2020)(Ahmed et al., 2021). The iron chelator dexrazoxane (ICRF-187) has been used to reduce doxorubicin-induced cardiotoxicity in mice, rats, rabbits, dogs, swine, and humans (Imondi, 1998; Kopp et al., 2019; Tahover et al., 2017). However, it is associated with hematological toxicities like neutropenia, leukopenia, anemia, thrombocytopenia and bone marrow suppression in patients (Tahover et al., 2017). The administration of zinc or bismuth has been reported to protect against doxorubicin-induced myocardial toxicity by the induction of metallothionein (Satoh et al., 1998).
There has been a casual relationship between doxorubicin-induced lipid peroxidation and cardiotoxicity (Julicher et al., 1986) (Wenningmann et al., 2019). The doxorubicin accelerated lipid peroxidation in the heart tissue and oltipraz attenuated the lipid peroxidation in the rat heart. The antarth, bael, naringin, hesperidin and metformin have been reported to reduce lipid peroxidation in mice and rat hearts earlier (Abdel-Raheem and Abdel-Ghany, 2009; Jagetia et al., 2005; Jagetia and Reddy, 2014; Jagetia and Venkatesh, 2015; Zilinyi et al., 2018). Similarly, berberine treatment decreased lipid peroxidation in cultured cardiomyocytes (Xiong et al., 2018). The chia seed oil has been reported to reduce doxorubicin-induced lipid peroxidation in rat serum (Ahmed et al., 2021).
The exact mechanism of protection afforded to rat hearts by oltipraz is not known. The doxorubicin-induces reactive oxygen species and removal of reactive oxygen species and attrition in lipid peroxidation by oltipraz may be responsible for its cardioprotective effect. The neutralization of doxorubicin-induced free radicals by oltipraz may have protected rat hearts against the doxorubicin-induced toxicity of rats. This would have been made possible by increasing glutathione, glutathione-s-transferase, catalase and superoxide dismutase by oltipraz in the present study. Oltipraz has been reported to be a potent inducer of phase-II detoxifying enzymes earlier (Benson, 1993)(Miao et al., 2003). The oltipraz is reported to suppress NF-κB and activate Nrf-2 that may have increased the antioxidant status by elevating glutathione, glutathione-s-transferases, glutathione peroxidases, catalases and superoxide dismutases in the rat heart and protected against the doxorubicin-induced cardiotoxicity. The oltipraz also suppressed the activation of tumor necrosis factor (TNF-α) and interleukin (IL-1β) in the rat heart earlier (Tang et al., 2018) and a similar action can not be ruled out in the present study.
Conclusions
The doxorubicin administration induced cardiotoxicity in the rat hearts as evidenced by the increased CK-MB activity and lipid peroxidation. The oltipraz pretreatment reduced doxorubicin-induced CK-MB activity and lipid peroxidation indicating that it protected against cardiotoxicity. The increased levels of glutathione, glutathione-s-transferase, catalase and superoxide dismutase seem to alleviate doxorubicin-induced free radical production and protect rat heart against the doxorubicin-induced toxic effects. This would have been due to the suppression of NF-κB, TNF-α, and IL-1β and elevation of Nrf-2.
Acknowledgements
The financial support from the University Grants Commission, Government of India, New Delhi NON-SAP UGC Grant No. F4-10/2010(BSR) to Prof. Ganesh Chandra Jagetia to carry out the above study is thankfully acknowledged.
Statement of conflict of interest
The authors have no conflicts of interest statement to declare.