Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 13 - Issue 1 - 2026
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Vural Fidan1*, Handan Koyuncu2, Hayal Toktas3
1Department of Otorhinolaryngology, Eskisehir City Hospital, Eskisehir, Turkey
2Department of Otorhinolaryngology, Yunus Emre Hospital, Eskisehir, Turkey
3Department of Neurology, Atasehir Memorial Hospital, Istanbul, Turkey
*Corresponding author: Vural Fidan, Department of Otorhinolaryngology, Cavdarlar Street, Eskisehir, 26080, Turkey.
Received: May 21, 2021
Accepted: June 18, 2021
Published: June 28, 2021
Citation: Vural Fidan, Handan Koyuncu, Hayal Toktas “Impact of Ketogenic Diet Versus Regular Diet on Voice Quality of Patients with Multiple Sclerosis”. Clinical Case Reports and Clinical Study, 4(4); DOI: 10.61148/2766-8614/JCCRCS/079
Copyright: © 2021 Vural Fidan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Purpose: Diets which have influence on wellness could alter in their structure. Apart from weight control, different diets are used in the treatment of many diseases. Ketogenic diet(KD) has been ventured to remedy of neurological diseases and obesity. Multiple sclerosis(MS) is a complex progressive disease of the central nervous system and it has an influence on quality of voice. Voice Handicap Index (VHI) is an assay that presents data to clinical and physiological appraisal about voice. We measured the effect of KD and RD on voice quality(VQ).
Methods: Sixty-eight subjects with MS who narrated a voice disorder associated to their disease were indiscriminately distributed to the KD or regula diet(RD) groups. We studied the VHI alter of patients before and 3 months after diet.
Results: Sixty-five MS subjects accomplished the study. Baseline VHI rates did not diverge seriously between groups. In the KD group, statistically improvement was seen in all VHI parameters 3 months after diet (p˂ 0.001).
Conclusion: Presently there are many therapies that aim speech and voice disorders in patients with MS. As such KD may be an option therapy to renovate VQ of patients with MS. A bigger sample size is required to find the function and pathophysiology of KD on VQ of MS patients.
Introduction
Multiple sclerosis (MS) is a demyelinating, autoimmune central nervous system disease with unknown etiology. It is believed that the immune system attacks and damages the myelin sheath of nerves. MS is characterized with with varied symptoms, including dysarthria and cognitive and linguistic impairments (1, 2). The prevalence estimates of MS vary geographically. MS generally initiates between the 2nd -4th decade. Motor speech difficulties are thought to be exhibit in around 40-50% of patient with MS (3).
The pattern of dysarthria alters, exhibiting the distinct neurological profile, but ataxic and spastic constituents are most common (4, 5). A diversity of drugs and therapies remain, that are devised with the aim of ameliorating signs, retarding impacts of the disease, and ameliorating quality of life (6). But the medications which are used to manage MS have side effects, spreading from minor problems such as fever, nausea to more severe effects, including Cushing syndrome (7). In reaction to persisting side effects of these drugs, many patients with MS are determining different procedures to handle and control signs (8).
Numerous scientific reports demonstrated the beneficial effects of diets on health and different types of diets have been used successfully in patients with many different disorders. Attention in diets has increased in the last decade. Ketogenic diet (KD) which contains high‐fat, low‐carbohydrate and adequate protein is utilized in patients with treatment of neurologic diseases (9, 10). KD has been trialled in unusual diseases such as enzyme deficiencies, cancer and diabetes (11). There are many surveys that have showed the positive impacts of KDs on a different of neurological disorders (12). A regular diet (RD) is a healty meal which contains variety of foods without any restriction (13). Procedures such as laryngeal endoscopy, patient-reported appraisals are also administered to evaluate voice problems (14). Appraisal of the patient’s self-perception is extremely important, and the extent of their speech and voice disorder should also be measured. The effect of a speech and voice disorder on a patient’s quality of life is subordinate on distinctive factors that differ from person to person and is the most important side of the appraisal (15). There are distinctive patient-reported determination procedures for speech and voice disorders, the most common patient-reported scoring system is the Voice Handicap Index-10 (VHI-10; range, 0-40, with higher scores indicating greater voice-related handicap ) (16). To date, there are no reported surveys that have investigated the impact of diet on speech and voice disorders in MS.
Therefore, the primary aim of this paper was to associate the effect of RD and KD on speech and voice disorders in subjects with MS measured by scores on the VHI-10.
Method
Sixty-eight subjects with MS (23 males, 45 females) with a speech and voice disorder who were drug-free between 2017 and 2019 enrolled in the study. The Local Ethics Committee approved the study. All patients presented written informed consent in accordance with the Declaration of Helsinki. Health and sociodemographic data of all subjects were registered in the report form, and all cases endured ear,nose and throat and neurologic analysis. MS was interpreted according to McDonald diagnostic criteria (17). On the VHI-10 form, patients were commanded to rate the applicable difficulties that patients with dysphonia can suffer in their routine life on a scale of 0–4 (0=never, 1=almost never, 2=sometimes, 3=almost always, 4=always). Statistical analysis was accomplished to resemble the degree of inquired variables before and 3 months after diet (Johns Hopkins protocol) (18). Median, standard deviation, lowest, highest, and ratio rates were utilized in the descriptive statistics of the data and statistical analyses were accomplished using SPSS for WINDOWS software (version20; SPSS Inc, USA). Division of patients by gender ratio and mean age was studied using the Pearson’s Chi-square and Mann–Whitney U tests. The Mann–Whitney U test was also used to correlate the VHI rates of the patients between the RD and KD groups. Test results were described as substantial for p< 0.001.
Results
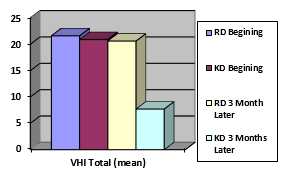
The 68 subjects were evenly seperated in two groups. Sixty-five MS subjects (22 males and 43 females) aged between 25 and 68 accomplished the research. Three subjects abandoned from the research. Diarrhea was observed in four patients (3 in KD, 1 in RD). Distribution by age and gender of patients whom accomplished the research are presented in Table 1. The mean total VHI-10 score at baseline in the RD group was 21.6±4.7; and in the KD group was 20.9±4.2. After 3 months, the mean total VHI-10 score of the RD group was 20.6±4.3; and the KD group was 7.7±1.4. Assignment of the mean VHI-ten scores in the two groups and statistically important deviations among the scores are shown in Table 2. When each argument was examined distinctly, scores for VHI-10 parameters were detected to be higher in the beginning versus 3 months after the KD (p< 0.001). (Table 2). Differences in Total VHI scores were shown in Figure 1.
|
|
Group |
||||
|
Regular Diet (n / %) |
Ketogenic Diet (n / %) |
Total (n / %) |
p |
||
|
Gender |
Male |
10 / 30.3 |
9 / 28.1 |
19 / 29.2 |
0.143 |
|
Female |
23 / 69.7 |
23 / 71.9 |
46 / 70.8 |
||
|
Age (Mean ± Standart Deviation) |
48.3±6.7 |
45.2±5.9 |
47.8± 6.1 |
0.125 |
|
Table 1: Demographic distribution and comparision of patients
|
|
|
p
|
|
|
||
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Discussion
MS is a demyelinating disease that is typically characterized by fatigue, imbalance, spasticity, chronic pain, cognitive impairment, bladder and bowel dysfunction, visual and speech impairments (19, 20). Speech and voice afflictions impact about 50-60% of MS patients, although the inherent pathophysiological appliances of these manifestations are not well understood. In spite of the high frequency of speech and voice symptoms in MS only too few of patients obtain speech intervention. Intervention modalities involve many pharmacological treatments (21). Steroid is noticed as the main drug for MS and signifcantly compresses all neurologic symptoms (22). KD is contained largely of fats and is attaining reputation as a nonpharmacological interference for neurologic disorders (23). Excessive-fat diet distends activation of dopaminergic process in the central nervous system (24). Ketone bodies show therapeutic relevance in brain restoration (25).
Conclusion
KD may be respected as a substitute intervention for speech and voice complaints in MS subjects whom do not desire medical therapy. We need new studies with a larger sample size to define the pathophysiology contrivances of this result.
Abbreviation
MS: Multiple sclerosis
KD: Ketogenic diet
RD: Regular diet
VHI-10: Voice Handicap Index-10
Declarations
Acknowledgments: We would like to thank Mr. Ersem Giritli for providing the information transmission in the research in the study.
Funding: No funding was used for the preparation and publication of the study.
Conflicts of interest/Competing interests: All authors do not have any conflicts of interest to declare.
Availability of data and material: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability: SPSS for WINDOWS software (version20; SPSS Inc, USA) was used for statistical analaysis.
Authors' contributions:
Vural Fidan has made a substantial contribution to the designing concept, collecting sources, analyzing and writing paper.
Handan Koyuncu has made a substantial contribution to the designing concept, collecting sources, analyzing and writing paper.
Hayal Toktas has made a substantial contribution to the designing concept, collecting sources, analyzing and writing paper.
Ethics approval: Ethical approval was taken Ethics Commitee of our hospital at 2020 year.
Consent to participate: Written informed constent was taken from all subjects.
Consent for publication: We, all authors, give our consent for the publication of identifiable details, which can include photograph(s) and/or videos and/or case history and/or details within the text (“Material”) to be published in the article. We confirm that we have seen and been given the opportunity to read both the Material and the Article (as attached) to be published by this journal.