Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 12 - Issue 1 - 2025
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
M.Sojka1, Z. Pawlak2*
1Kujawy University, Mechanical Department, Hallera 32, 86-300 Grudziadz, Poland and CORSAR Engineering Industry, Glogowa 2, 86-031 Osielsko, Poland
2Tribochemistry Consulting, Salt Lake City, UT 84117, USA and University of Economy, Biotribology Lab, Garbary 2, 85-229, Bydgoszcz, Poland
*Corresponding author: Z. Pawlak, Tribochemistry Consulting, Salt Lake City, UT 84117, USA and University of Economy, Biotribology Lab, Garbary 2, 85-229, Bydgoszcz, Poland Grudziadz, Poland and CORSAR Engineering Industry, Glogowa 2, 86-031 Osielsko, Poland
Received: May 27, 2021
Accepted: June 06, 2021
Published: June 09, 2021
Citation: M.Sojka, Z. Pawlak. “Lubricin and Hyaluronan Cannot Support Cartilage Lubrication Without Active Phospholipids in Osteoarthritic and Rheumatoid Arthritis Joints”. Clinical Case Reports and Clinical Study, 3(4); DOI: 10.61148/2766-8614/JCCRCS/075
Copyright: © 2021 Z. Pawlak. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium,provided the original work is properly cited.
This article focuses on disruptive action of the three components present in the synovial fluid: hyaluronan, lubricin and phospholipids, present in active osteoarthritis (OA) and rheumatoid arthritis (RA). Phospholipids lamellar phase adsorbed to hyaluronan and lubricin are two important components in the synovial fluid that both have been suggested to facilitate the lubrication of synovial joints. Recently examined synovial fluid (SF) for the concentration of phospholipids in osteoarthritic by Kosinska et. al., remain in surpassingly high concentration. The lubricating properties of biomolecules with deactivated phospholipids, hyaluronan and lubricin have been shown to not have the same lubricating ability as normal synovial fluid. Based on these observations, we conclude that deactivated phospholipids indeed are unable to provide low friction forces and high load bearing capacity.
Introduction
Phospholipid bilayer structures on the surface of cartilage are a main lubricant in joints [1]. The multilamellar structure of phospholipid, namely the surface amorphous layer (SAL) was identified as phosphatidylcholine (41%), phosphatidylethanolamine (27%) and sphingomyelin (32%) by Sarma et al., with unsaturated phospholipids content more than 50% [1]. The phospholipid bilayers and synovial fluid are affected by osteoarthritis and rheumatoid arthritis [2, 3].
Main destruction in osteoporosis disease is deactivation of PLs molecules in synovial fluid and phospholipid from bilayers. Therefore, when joint is under osteoporosis disease: (a) Deactivated PLs can’t form liposomes, PLs phases or bilayers, (b) Deactivated PLs molecules lost ability to adsorb hyaluronan and lubricin molecules, (c) Lubricin and hyaluronan can’t take leading role being a lubricants without active phospholipids, PLs [2, 3, 4]. Phospholipid molecules by self-organization process form bilayers and contribute to cartilage boundary lubrication [5, 6]. Also, the adsorption of PLs by hyaluronan and lubricin and their supportive function in cartilage boundary lubrication remain accepted [5]. A model that was developed and introduced by Hills [5] with a PLs membrane-like structure adhering to the surface of articular cartilage was confirmed by us [6] and by many laboratories [1, 2, 4, 5].
In this work we focus on interactions between three major components of synovial fluid: hyaluronan, lubricin and phospholipids. A surface amorphous layer (SAL) covers the surface of cartilage, and its importance for lubrication has also been pointed out [1, 6]. However, it has been clearly shown that hyaluronan and lubricin does not provide low friction and does not work as boundary lubricants in osteoarthritis (OA) and rheumatoid arthritis (RA) conditions [3, 4].
Materials and methods
The articular cartilage specimens were collected from bovine knees aged 15-20 months. Osteochondral plugs, of 5 and 10 mm in diameter, were harvested from lateral and medial femoral condyles using a circular stainless steel cutter. The cartilage discs were cut into 3-mm plugs with underlying bone. Two types of samples were tested: untreated bovine cartilage and bovine cartilage treated with a Folch reagent a lipid-rinsing solution (a 2:1 v/v mixture of chloroform and methanol). Cartilage samples were immersed in a lipid-rinsing solution to remove the lipids for 2 min (partially PL depleted) and 21 min (totally PL depleted) from the surface of the cartilage [6]. After preparation, the specimens were stored at 253 K in a 0.155 M NaCl (pH = 6.9) solution and fully defrosted prior to testing. The cartilage discs were then glued to the disc and pin stainless steel surfaces, and friction tests were conducted in the in a 0.155 M NaCl.
Friction test: The measurements were performed using a sliding pin-on-disc tribotester T-11 manufactured by ITeR, Poland., see Fig.1. The tests were conducted at room temperature, at a speed of 1 mm/s during 1, 3, 5, 7 and 10 minutes under a load of 15 N (1.2 MPa) which correspond to the physiological lubrication condition. A total number of five tests were performed using fresh sample from each experimental specimen and set up.
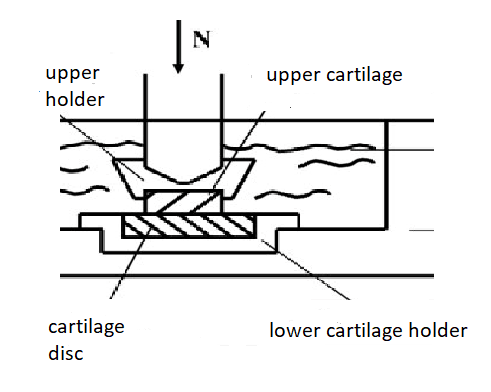
Figure 1: Schematic diagram of the friction test apparatus
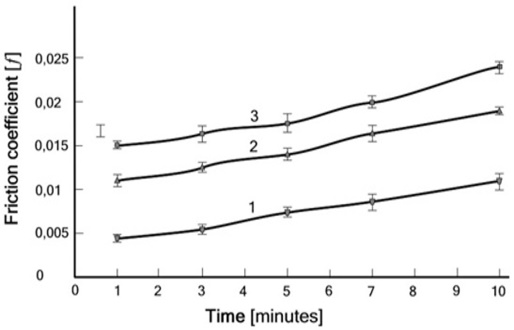
Figure 2: Friction coefficient vs. time for the (cartilage/ cartilage) pair, (curve 1) normal sample, (curve 2) with early osteoarthritis (sample partially PL depleted) and (curve 3) late osteoarthritis (sample totally PL depleted).
Tests of the friction coefficient vs. time for the (cartilage/cartilage) pair, (curve 1) normal one and (curve 2) with early osteoarthritis and (curve 3) late osteoarthritis have demonstrated that the decrease of PLs species in OA is a mechanism to increase friction, see Fig. 2
|
|
Figure 3: 3D topograpicical image atomic force microscopy of (A) normal healthy cartilage after image processing, showing the nanostructure arrangement of the surface amorphous layer with several peaks and troughs (B) delipidized cartilage after image processing, indicating the loss of the membranous overlay (surface amorphous layer) of the articular surface [(length (X) and breadth (Y) of the scanned area, and average peak height of SAL [6].
In the previous study, we established using a high-resolution imaging technique, atomic force microscopy (AFM), that a well-organized surface-active phospholipid covers the surface of the cartilage (SAPL) layer in a lamella-like arrangement, as previously described by Hills [9] (Fig. 3A). Wiping of the articular surface with a lipid rinsing reagent resulted in a drastic removal of the SAL (Fig. 3B). In mammals, the intact lipid layer of cartilage is lost during degeneration, thus affecting the efficient lubrication of the joint.
Results and discussion
Interactions between phosphate functional group (PO4-) and protonated amine (NH3+) functional group of β2-GPI (-NH3+) have been established to occur in the identified molecular complexes β2-GPI (-NH3+) (-PO4-) PL. The molecular complexes formed in osteoarthritic joints depend on the acid-base character of the reacting molecules [5]. The protonated amine functional groups from β2-GPI (-NH3+) are engaged in electrostatic interactions with PL(-PO4-) phosphate of phospholipids and complex formation, β2-GPI (-NH3+) (-PO4-) PL) [6, 7, 8].
Bilayer phospholipid molecules attached by SF at pH ~7.4 are in anionic form with functional group (-PO4-). Such functional group has ability to interact electrostatically with antibody molecule (β2-Glycoprotein I), (β2- GPI) with functional (-NH3+) group with association constant Kassoc~ 105 [6].
PL-(-PO4-) + β2-GPI (-NH3+) → β2-GPI (-NH3+) (-PO4-) PL); Kassoc~ 105 (1)
|
(A) closed form (B) open hockey-stick (C) (D) |
Figure 4: (β2-Glycoprotein I) in closed form (A), into an open hockey-stick-like conformation (B), each molecule has five domains (1-5). The binding site (B5) is accessible to the autoantibodies. The phospholipid bilayer (C), and the open hockey stick-like (Β2-GPI) binding to negatively charged phospholipids (D).
Β2-Glycoprotein I, (Β2-GPI) (MW of 50 kDa) circulates in the body and autoimmune disease transforms Β2-GPI (closed form) in antibody (open hockey-stick), Fig. 4. [7, 8]. The (Β2-GPI) participates in the antiphospholipid antibody syndrome (APS) through binding of β2- GPI to the anionic charged phospholipid (-PO4-) functional group. In plasma, it occurs as a closed circular protein in which domain 1 interacts with domain 5 (Fig. 4A). In osteoarthritic joint the (β2-Glycoprotein I), (Β2-GPI) binding site in domain 5 (Fig. 4B) contains 326 positively charged amino acids functional group (-NH3+) at pH ~7.0 [7, 8]. The open hockey stick-like conformation β2-GPI is complexed to negatively charged phospholipid functional anionic group (-PO4-) resulting in the destruction of bilayers and deactivation of phospholipid molecules in synovial fluid (Fig. 1C, D). Product of association (reaction1) has no ability form liposomes, PL phases and bilayers and rebuilds PLs membrane. Adsorption process biomacromolecules (lubricin and hyaluronan) do not exist when functional group (-PO4-) is already occupied by the protonated amino functional group (-NH3+) of (Β2-GPI). The concentration of hyaluronan in OA SF declines to 0.1–1.3 mg/ml, compared with 1–4 mg/ml in healthy controls [2, 3]. Hyaluronan with adsorbed phospholipids contributes to lowering friction by altering sliding to rolling action.
Hyaluronan, lubricin, and phospholipid species (PLs) contribute independently or together to the boundary lubrication of articular joints that is provided by synovial fluid (SF).
Lubricin is a surface-active mucinous glycoprotein secreted in the synovial joint that plays an important role in cartilage integrity. In healthy joints, in presence of surface active PLs lubricin molecules adsorb the cartilage surface, supporting boundary lubrication [10, 11]. Lubricin was found to be ineffective in reducing friction in arthritic articular cartilage [12].
 (A)
(A) 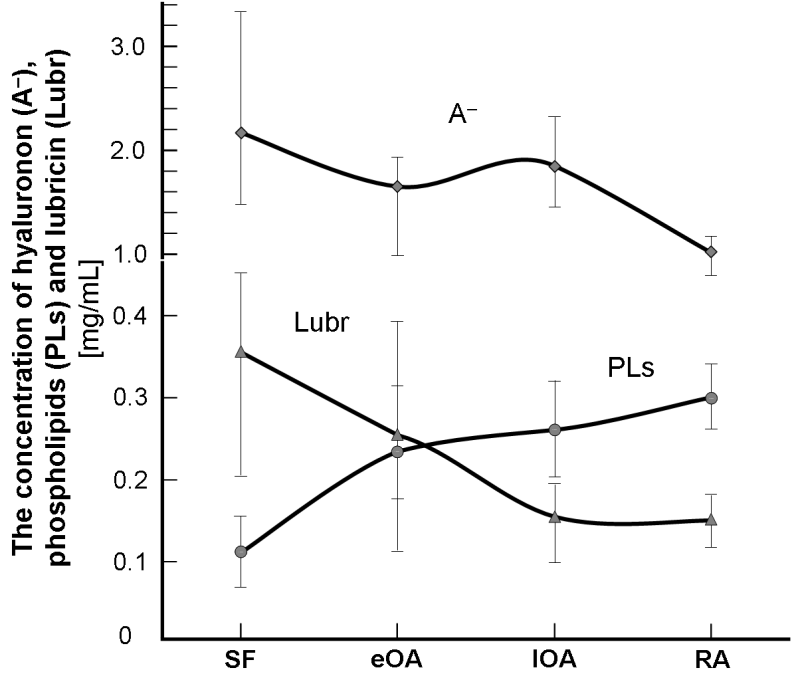 (B)
(B)
Figure 5: (A) Surface of articular cartilage under the wet (hydrophilic) and air-dry conditions (hydrophobic). Book cover “Articular Cartilage: Lamellar-Repulsive Lubrication of Natural Joints” [6]. (B) (B) Hyaluronan (A-), phospholipids (PLs) and lubricin (Lubr) in human synovial fluid (SF) in patients with healthy joints control (SF) and joint diseases with early osteoarthritis (eOA), late osteoarthritis (lOA) and rheumatoid arthritis (RA) [2, 3].
Variations in surface energy lead to transformation cartilage surface from bilayer (hydrophilic) to monolayer (hydrophobic) [6], Fig.5. The concentration of components of synovial fluid (SF), such as hyaluronan (A-), lubricin, and surface-active phospholipids, from unaffected controls (or normal), eOA, lOA, and RA in the human synovial fluid are shown in Fig. 5 (B). During osteoarthritis (OA), and rheumatoid arthritis (RA), SF contain less hyaluronan (A-), and lubricin, but 2 to 3 times more with phospholipids. Also, the MW distribution of (A-) shifted toward the lower range in OA and RA SF. These results indicate that activities in OA and RA SF are enhanced, leading to decreased levels of lubricin and high-MW hyaluronan (A-).
Conclusion
Lipids in osteoarthritic cartilage lost surface active properties by being involved in the interaction of enzymatically activated β2-Glycoprotein I and complex formation, β2-GPI (-NH3+) (-PO4-) PL. In conclusion, we found that both macromolecules hyaluronan and lubricin in osteoarthritic and rheumatoid arthritis cannot support lubrication when surface active PLs are deactivated.
There is evidence that the frictional behavior of the solid bilayer lubricant is dominated by lamellar slippage of bilayers, the high concentration of PL during inflammation (APS) being of secondary importance. The phospholipids content in synovial fluid (SF) during joint inflammation, osteoarthritis and rheumatoid arthritis is significantly higher (2 to 3 times) above the normal concentration of PL and being deactivated (β2-GPI-NH3+) + (PLs–PO4-) → β2-GPI(-NH3+ PO4--PL) has a poor boundary-lubricating ability. Deactivated PL molecule is unable to form bilayers, lamellar phases, and liposomes. Our hypothesis about cartilage degradation of PL bilayers by antibodies β2-Glycoprotein I is discussed considering antiphospholipid syndrome (APS). From osteoarthritic joints study we learned about the importance of phospholipids in friction reduction not only in joints but in the whole human and animal lubrication system.
Conflict of interest: The author(s) declare that they have no conflicting interests.
Funding: This study was funded by an internal Tribochemistry Consulting grant.
Acknowledgements: We would like to thank Ms Joanna Pawlak for editing Ms and critical review.