
Francesco Fioretti1,2, MD, Andrea Dell’Aquila1,2, MD; Dario Salvatore Cani2, MD; Alberto Madureri2, MD; Savina Nodari1,2*, MD.
1 Section of Cardiovascular Diseases, Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, Italy
2 Cardiology Unit, ASST Spedali Civili di Brescia Hospital, Brescia, Italy
*Corresponding author: Savina Nodari, Associate Professor of Cardiology Section of Cardiovascular Diseases, Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, Italy.
Received: March 24, 2021
Accepted: April 06, 2021
Published: April 20, 2021
Citation: Francesco Fioretti, Andrea Dell’Aquila, Dario Salvatore Cani, Alberto Madureri, Savina Nodari, “Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) related Myocarditis and Pericarditis in a Pediatric Patient: A Case Report Providing New Insights On The Coronavirus Disease 2019 (Covid-19) Pandemic.” Clinical Case Reports and Clinical Study, 3(3); DOI: 10.61148/2766-8614/JCCRCS/048
Copyright: © 2021 Savina Nodari. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Myocarditis is often caused by viral infection. However, few cases of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) related myocarditis are described in literature, and, to our knowledge, few pediatric cases are reported. We report the case of a 16 years old male who tested positive for SARS-CoV-2 infection and developed myopericarditis, confirmed by cardiovascular magnetic resonance imaging. Respiratory involvement was excluded by chest computed tomography. Nasopharyngeal swabs (NPS) for SARS-CoV-2 were negative, however plasmatic antibody (Ab) assay was positive. Clinical conditions of the patient improved rapidly after treatment with ibuprofen, hydroxychlorokine and enoxaparin. This case highlights cardiac involvement as a complication of SARS-CoV-2 infection, even in the absence of respiratory involvement. Elderly are more at risk of infection, but pediatric patients might be affected as well. NPS may be negative, especially without respiratory involvement, therefore Ab testing might be of paramount importance to reach diagnosis in these cases.
Introduction
A new form of viral pneumonia caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1], starting at the end of December 2019 in China, rapidly diffused globally reaching a pandemic scale [2]. Italy, and in particular the Lombardy region, was one of the first and most affected areas worldwide [2,3]. The clinical course of the disease, named Coronavirus Disease 2019 (COVID-19), is mostly characterized by respiratory tract symptoms, including fever, cough, and fatigue, and complications related to pneumonia and acute respiratory distress syndrome (ARDS) [4]. Cardiovascular diseases make individuals more susceptible to COVID-19 and its complications [4], and direct and indirect cardiovascular damage is not uncommon [5,6]. Only few cases of SARS-CoV-2-related myocarditis are described in literature7–11. We hereby present a clinical case of SARS-CoV-2 related myocarditis and pericarditis, which, to our knowledge, is one of the first cases reported in a pediatric patient (pt).
Case Report
In April 2020 a 16 years old white male without history of heart disease presented himself to the Emergency Department of a hospital in the suburban area of Brescia, Lombardy, Italy, reporting fever lasting for a week, with body temperature (BT) >39° C, poorly responsive to acetaminophen, associated with chills, diarrhea and fatigue. He or she was treated with azithromycin without any clinical improvement. The pt never presented cough or dyspnea. However the father of the pt had fever and similar symptoms in the previous week, as well as ageusia and anosmia, and was
isolated for suspected COVID-19.
Physical examination revealed blood pressure (BP) 90/60 mmHg, heart rate (HR) 104 bpm, respiratory rate (RR) 19 Bpm, oxygen saturation (SpO2) while breathing in ambient air (AA) 99%, and BT 38.9° C. Pericardial rubs were present at cardiac auscultation.
Arterial blood gas analysis AA demonstrated arterial oxygen partial pressure 90.7 mmHg, ratio of PaO2 and fractional inspired oxygen 436 mmHg, pH 7.51, arterial carbon dioxide partial pressure (PaCO2) 30.9 mmHg, and bicarbonate concentration 24.2 mEq/L.
Electrocardiogram (ECG) showed sinus tachycardia, incomplete right bundle branch block, slight ST segment depression (1 mm) in inferior leads (II, III, aVF) and slight ST segment elevation (1 mm) in aVR (Fig. 1/A).
Full blood count (FBC) showed leukocytosis with lymphopenia. Plasmatic levels of D-dimer, fibrinogen, ferritin, C-Reactive Protein (CRP) and high-sensitivity Troponin-T (TnT) were elevated (Tab.1).

Fig. 1 Electrocardiogram and Chest Computed Tomography Findings. (A)
Electrocardiogram at admission showed sinus tachycardia (heart rate of 104 bpm), incomplete right bundle branch block, slight ST segment depression (1 mm) in inferior leads (II, III, aVF), slight ST segment elevation (1 mm) in aVR, and T-wave inversion in V1. (B) Contrast-Enhanced and Non-Enhanced Chest Computed Tomography excluded pulmonary embolism and revealed mild circumferential pericardial effusion, more prominent on the right ventricular side (maximum diameter of 1 mm).
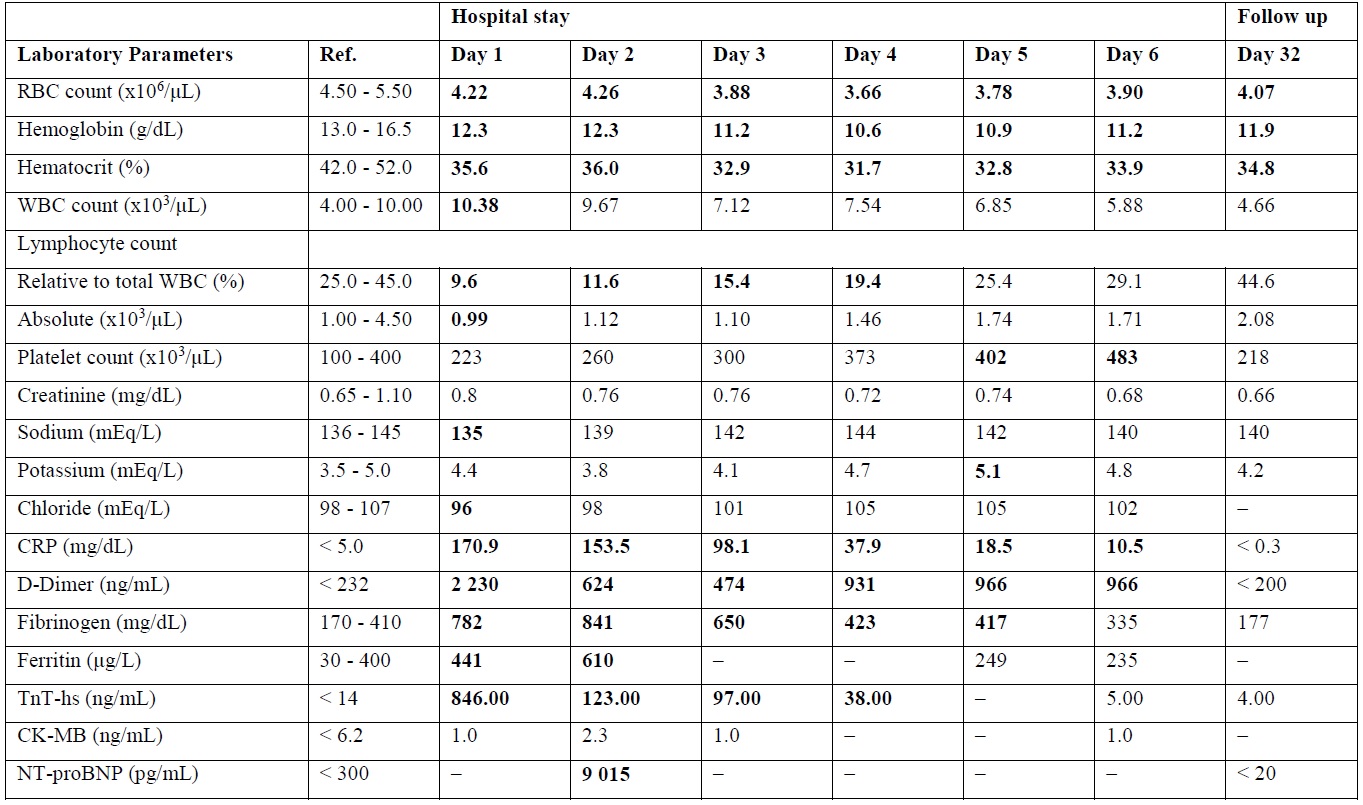
Tab. 1 Blood Tests Laboratory Parameters.
Values in bold are outside normal range. –: Not Applicable; CK-MB: Creatin Kinase MB (Myocardial Band) isoenzyme; CRP: C-Reactive Protein; NT-proBNP: N-Terminal Pro-Brain Natriuretic Peptide; RBC: Red Blood Cell; Ref.: Reference Values; TnT-hs: High Sensitivity Troponin T; WBC: White Blood Cell.
Transthoracic Echocardiography (EchoTT) revealed left ventricular (LV) diffuse wall with ejection fraction (EF) 40%, right ventricular (RV) dilation and dysfunction with Tricuspidal Annular Plane Systolic Excursion (TAPSE) 17 mm, and presence of mild circumferential pericardial effusion.
In the suspect of SARS-CoV-2 related myopericarditis, the pt was immediately transferred to the Inpatient Cardiology Department of our center. To exclude pulmonary embolism and pneumonia, contrast-enhanced and nonenhanced chest computed tomography exam was performed in the same day of admission, showing absence of both pleuro-parenchymal lesions and pulmonary embolism, but presence of circumferential pericardial effusion (Fig. 1/B). Seriated blood test showed TnT maximum concentration (max) 846 ng/L, max Creatin-Kinase MB isoenzyme (CK-MB) 2,3 mcg/L, and max N-terminal pro–brain natriuretic peptide (NT-proBNP) 9 015 ng/L (Tab.1).
Research of SARS-CoV-2 RNA with Polymerase Chain Reaction technique on multiple nasopharyngeal swabs (NPS) was negative. However plasmatic antibody (Ab) assay was positive for SARS-CoV-2 infection. Subsequent Ab testing for cardiotropic viruses (Coxsackie, Epstein-Barr Virus, Cytomegalovirus, Herpes Simplex 1 and 2, Herpes Zoster, HIV, Parvovirus B19, Echovirus, Influenzavirus A and B, Adenovirus, Respiratory Syncytial Virus) excluded recent viral infection. Multiple hemocoltures for fungi and aerobic and anaerobic bacteria were negative.
Cardiac Magnetic Resonance imaging was performed 5 days after admission, showing biventricular dilation with normal systolic function, with LV End-Diastolic Volume (EDV) 220 mL (116 mL/m2), LV End-Systolic Volume (ESV) 96 mL (51 mL/m2), RVEDV 223 mL (117 mL/m2), RVESV 103 mL (54 mL/m2), LVEF 57% and RVEF 54%, and circumferential pericardial effusion. There was diffuse LV edema on Short- Tau Inversion Recovery images, and evidence of LV intramural late gadolinium
enhancement of the antero-lateral, inferior and infero-lateral segments (Fig. 2).
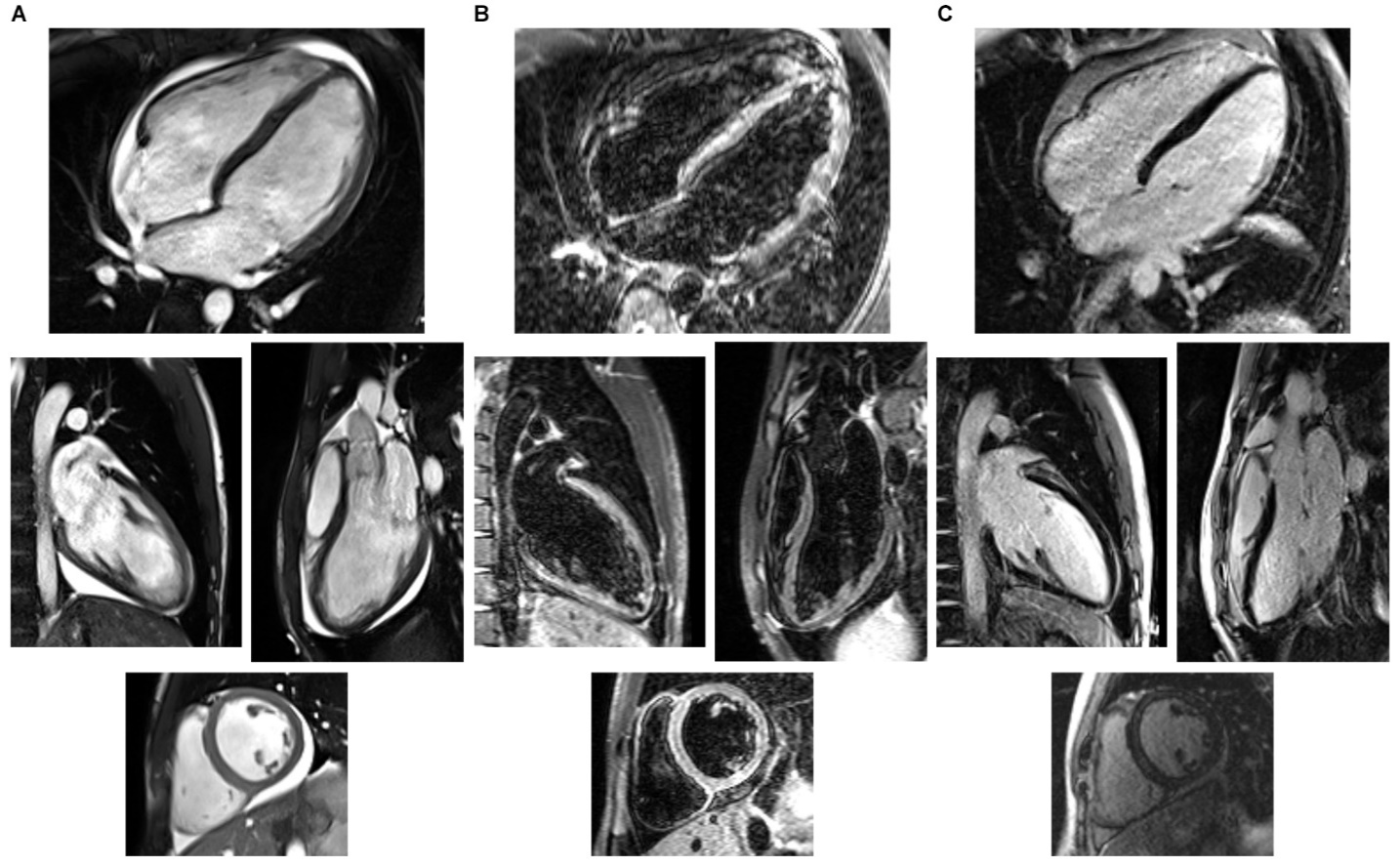
Fig. 2 1.5-Tesla Cardiac Magnetic Resonance Imaging Findings.
(A) Cine images obtained from Steady-State Free Precession sequences, frozen on the end-diastolic frames. (B) Images obtained from Short-Tau Inversion Recovery sequences, showing diffuse myocardial signal hyperintensity, suggesting interstitial edema. (C) Images obtained from Phase-sensitive Inversion Recovery sequences, showing left ventricular subepicardial intramural late gadolinium enhancement of the antero-lateral segments on the medio-basal level and intramural enhancement of the infero-lateral basal segment. All images show circumferential pericardial effusion, more prominent on the right ventricular side and around the inferior segment of the left ventricle. On each panel (A,B,C), from the top, in clockwise order: 4-chamber view, long-axis view, short-axis view at basal level, 2-chamber view images.
Continuous ECG monitoring during hospital stay showed no arrhythmias. Treatment with ibuprofen, hydroxychlorokine, subcutaneous enoxaparin and empiric antibiotic treatment (azithromycin and ampicillin/sulbactam) was started at admission and continued throughout hospital stay.
Clinical conditions of the pt improved rapidly. The pt was discharged 6 days after admission free of symptoms, apyretic and with normal vital signs (BT 36° C, RR 12 Bpm, BP 110/70 mmHg, HR 53 bpm, SpO2 AA 99%), with the following therapy: Ibuprofen 600 mg tid, Colchicine 0.5 mg qd.
FBC was normal, plasmatic acute phase proteins and myocardial necrosis markers decreased, while D-dimer levels remained high (Tab. 1). ECG revealed resolution of previously described ST segment abnormalities, echoTT showed mild pericardial effusion.
A week later the pt was evaluated at the Cardiology Outpatient Clinic, reporting slight fatigue. EchoTT revealed persistence of pericardial effusion, therefore continuation of treatment with Ibuprofen and Colchicine was suggested. A month after admission, the pt was evaluated again at the Cardiology Outpatient Clinic, reporting no symptoms. FBC, myocardial necrosis markers, D-dimer, CRP and NT-proBNP were all within reference values (Tab.1).
EchoTT highlighted persistence of LV dilation, with LVEDV 185 mL (97 mL/m2), LVESV 92 mL (48 mL/m2) and LVEF 50%, normal RV size and function (TAPSE 23 mm, RV s’ velocity at Tissue Doppler Imaging 12 cm/sec, RV fractional area change 45%). Although reduced, pericardial effusion was still present around the RV. Therefore continuation of therapy with Ibuprofen and Colchicine was suggested, and a subsequent appointment at the Cardiology Outpatient Clinic was scheduled three weeks after.
Discussion
To our knowledge, this is one of the first cases reported of SARS-CoV-2 related myocarditis and pericarditis in a pediatric pt.
Limitations of this report include lack of histological demonstration of myocarditis and viral genome presence in the heart, as endomyocardial biopsy was not performed.
However the association of clinical presentation, imaging findings, blood test results, pt social contacts and response to treatment supports the formulated diagnosis [5–11].
Direct and indirect SARS-CoV-2 mediated cardiac damage is not uncommon [5,6]. Cardiovascular disease is linked to the pathogenesis of COVID-19 by the renin-angiotensin-aldosterone system and, in particular, by the Angiotensin- Converting Enzyme 2 (ACE2). This molecule acts as a viral receptor (SARS-CoV-2 binds with ACE2 through glycoprotein spyke S, which grants cell entry) [5,6,12] and seems to play a pivotal role in cardiovascular damage [5,6].
It should be noted that many aspects of disease severity are unique to COVID-19 and are rarely observed in other respiratory viral infections, such as lymphopenia13,14 and cytokine storm leading to ARDS, cardiovascular damage and multi-organ failure [5,6,13].
With this case report the Authors want to convey the following messages:
1. Elderly are more at risk of SARS-CoV-2 infection, complications and death [4], but pediatric pts are not immune to COVID-19.
2. Sometimes infected pts show no respiratory symptoms, or the first phase of the respiratory tract infection may be asymptomatic [4].
3. NPS may be negative, especially if respiratory involvement is absent, therefore it is important to consider clinical presentation and pts social contacts (especially in family and work-related settings). Serological Ab testing might be of paramount importance to reach diagnosis in these cases [15], although future studies are needed to clarify its real clinical value.
4. Cardiovascular disease is not only a risk factor4, but might also be a consequence of the infection, worsening prognosis in affected pts [5,6].
Highlights
· Case report of pediatric SARS-CoV-2 related myocarditis and pericarditis
· SARS-CoV-2 infection may affect the heart, even without respiratory involvement
· Elderly are more at risk of infection, but pediatric patients may be affected
· Nasopharyngeal swabs may be negative, especially without respiratory involvement
· Antibody testing might be crucial to diagnose cases without respiratory involvement
Acknowledgements
None.
Source of Funding
None.
Declaration of Interest
None.