Clinical Case Reports and Clinical Study
OPEN ACCESS | Volume 13 - Issue 1 - 2026
ISSN No: 2766-8614 | Journal DOI: 10.61148/2766-8614/JCCRCS
Shreya Podder MD1*, Christopher S. King MD1, Erik Osborn MD1, Aldo Iacono, MD2, James Lantry MD2, Mehul Desai MD1
1INOVA Fairfax Medical Center, Fairfax, VA
2University of Maryland Lung Transplant, College Park, MD
*Corresponding authors: Shreya Podder, INOVA Fairfax Medical Center, Fairfax, VA
Received: March 08, 2021
Accepted: March 16, 2021
Published: March 19, 2021
Citation: Shreya Podder, Christopher S. King, Erik Osborn, Aldo Iacono, James Lantry, Mehul Desai. “Irreversible Respiratory Failure in Healthy Individuals Exposed to Trimethoprim-Sulfamethoxazole –Case Report”. Clinical Case Reports and Clinical Study, 5(2); DOI: 10.61148/2766-8614/JCCRCS/035
Copyright: © 2021 Shreya Podder. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background
Respiratory failure secondary to Trimethoprim-Sulfamethoxazole (TMP/SMX) toxicity has been described, with prior case series demonstrating resolution with discontinuation of the drug, steroid administration and temporary ventilatory support. We describe TMP/SMX associated severe ARDS in an adolescent and an adult patient who required extracorporeal membrane oxygenation (ECMO).
Case presentation
The adolescent required lung transplantation and the adult expired despite ECMO support due to fungemia. Both patients presented initially with mild respiratory symptoms and fever following treatment of skin infections with TMP/SMX. Notably, both were complicated by pneumomediastinum and had diffuse alveolar hemorrhage (DAH) on bronchoscopy prior to being placed on ECMO. TMP/SMX has not been explicitly linked with DAH, although its association with multiple vasculitis syndromes has been described previously. Despite optimal medical therapy, both patients developed irreversible hypoxemic respiratory failure due to TMP/SMX-associated pneumonitis.
Conclusion
Our case series adds to a limited set of cases that demonstrate failure of resolution of acute respiratory distress syndrome (ARDS) with conventional medical therapy, and to our knowledge, is the first report of an adult requiring venovenous extracorporeal support for severe TMP/SMX related lung injury.
Introduction
Use of oral and intravenous TMP/SMX is known to cause a variety of adverse immunologic effects. However, primary respiratory involvement remains a rare presentation. TMP/SMX has been associated with various presentations of acute lung injury, including acute fibrinous organizing pneumonia, eosinophilic pneumonia and subacute pneumonitis (1,2,3). Diagnosis of primary respiratory failure secondary to TMP/SMX is a diagnosis of exclusion, requiring evaluation of infection, immunologic disease, alternate drugs and a correlation between drug exposure and presentation of symptoms. Prior case reports have described resolution of pulmonary disease with discontinuation of TMP/SMX and initiation of systemic steroids. The pathophysiology of TMP/SMX induced lung injury remains poorly understood. We present two cases of previously healthy patients who developed TMP/SMX associated acute lung injury after being exposed to the medication. Both cases showed failure of resolution of acute lung injury despite discontinuation of TMP/SMX and optimal medical therapy.
Case 1
A 16-year-old otherwise healthy female presented to the hospital with acute onset chest pain and dyspnea. Four days prior to admission, the patient experienced lip swelling that she attributed to TMP/SMX, which she was taking for acne. She had taken single strength TMP/SMX tablets twice daily for ten days prior to stopping the medication. The following morning, the patient developed a fever with dyspnea. Rapid flu test and respiratory syncytial virus (RSV) panel were negative. She was sent to the emergency department, where her chest x-ray was concerning for pneumomediastinum. Computed tomography (CT) angiography of the chest demonstrated pneumomediastinum with bilateral ground glass attenuation, without evidence of pulmonary embolism (Figure 1). She was started on amoxicillin for suspected pneumonia. Her respiratory status continued to deteriorate, and a repeat CT chest showed worsening pneumomediastinum and pulmonary infiltrates (Figure 2). Due to inability to maintain adequate oxygenation, the patient was intubated on hospital day seven. Bronchoscopy after intubation revealed diffuse alveolar hemorrhage (DAH). Serologic evaluation for Goodpasture syndrome and ANA-associated vasculitis were negative. She was treated with solumedrol and underwent four sessions of plasmapheresis for presumed pauci-immune vasculitis secondary to TMP-SMX. The patient was transferred to an ECMO center as she was unable to adequately oxygenate and ventilate the patient with standard mechanical ventilation. The patient failed to recover despite prolonged ECMO support and eventually underwent successful bilateral lung transplant.
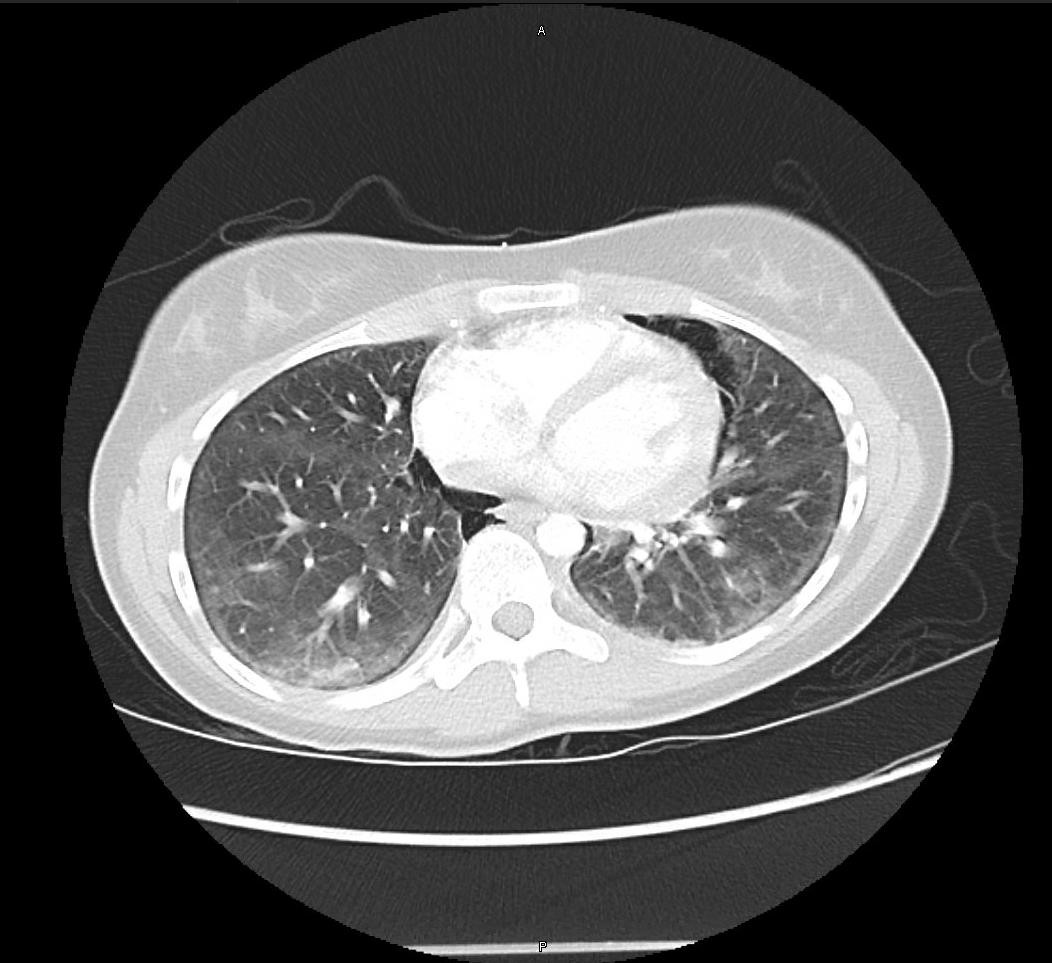
Figure 1: Evidence of pneumomediastinum. No pulmonary embolus. There are hazy and patchy aeration sections bilaterally with ground glass attenuation, with scattered atelectasis.

Figure 2: Increase in subcutaneous emphysema from previous CT angiogram, with persistent pneumomediastinum which has increased. Diffuse ground glass opacities and new reticulonodular interstitial opacities were also seen in the lung bases.
Case 2
A 29-year-old previously healthy male developed a superficial right lower abdominal skin abscess, which was treated with single strength TMP/SMX tablets taken twice daily. The abdominal abscess did not improve and cephalexin was added to the treatment regimen. Within a week of initiation of antibiotics, the patient developed substernal chest discomfort and dyspnea prior to developing a headache, followed by fever. Initial CT scan of his chest did not show any pulmonary infiltrates. A full evaluation for infection, including cultures (blood, urine, respiratory) and lumbar puncture did not show any evidence of acute infection. The patient’s dyspnea and chest pain progressed, and he was admitted for acute hypoxic respiratory failure, requiring intubation on hospital day two. A repeat CT chest showed concern for interstitial lung disease (Figure 3). Bronchoscopy showed bloody fluids concerning for DAH and cultures from bronchoalveolar lavage were negative. Patient was treated with methylprednisolone and underwent four treatments of plasmapheresis. Patient was successfully extubated one week after intubation and was started on a steroid taper. However, his oxygen requirement again increased after extubation and he required re-intubation on hospital day fifteen. Progression to acute respiratory failure lead to him being placed on venovenous ECMO. He was transferred to a transplant center for expedited work-up; however, the patient developed fungemia and unfortunately died from multi-organ failure.
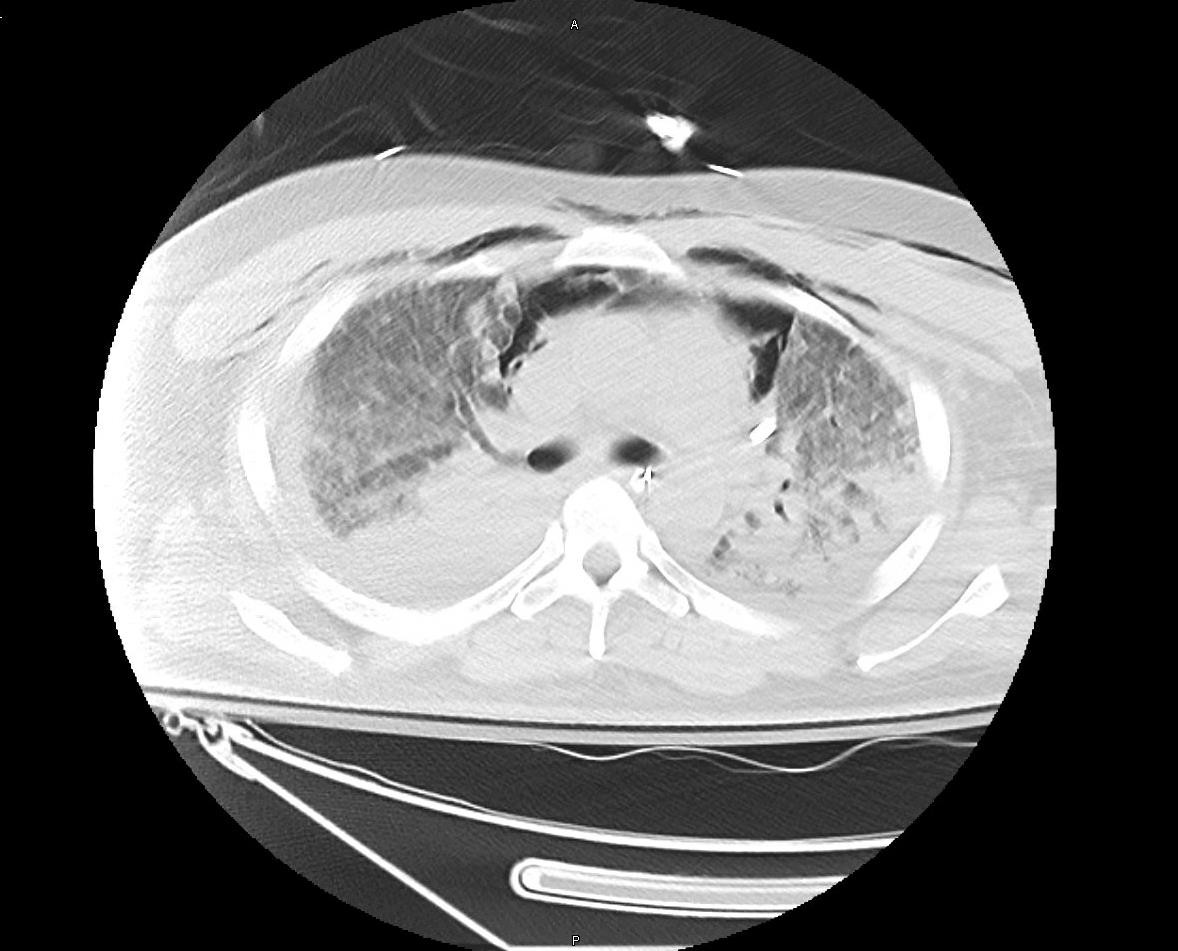
Figure 3: CT chest shows extensive bilateral consolidating pulmonary infiltrates in the right lung apex and in both lower lobes, concerning for pneumonia. Lung elsewhere shows ground glass opacities and scattered nodules without apparent honeycombing.
Conclusion
We reviewed two cases of previously healthy patients who received TMP/SMX prior to developing acute lung injury requiring mechanical ventilation. Both progressed to severe ARDS that required venovenous extracorporeal support. Work-up of these patients involved transfer to a tertiary center, with extensive evaluation for infectious, immunologic and rheumatologic etiologies of progressive respiratory failure. Case 2 underwent workup for Sjogren’s syndrome due to patient’s family history of biopsy proven
Sjogren’s syndrome and patient’s own positive serologies for anti-Ro (SSA) and anti-La (SSB). However, Sjogren’s syndrome did not fully explain his symptoms. Moreover, the most common pulmonary manifestation of Sjogren’s disease is nonspecific interstitial pneumonia, particularly the fibrosing variant (4). Based on the timeline and absence of alternate etiologies of lung injury, TMP/SMX was determined to be the cause. Both patients received pulse dose steroids and plasmapheresis. Despite aggressive medical therapy, both patients failed to demonstrate resolution with one requiring transplant and the other expiring secondary to fungemia.
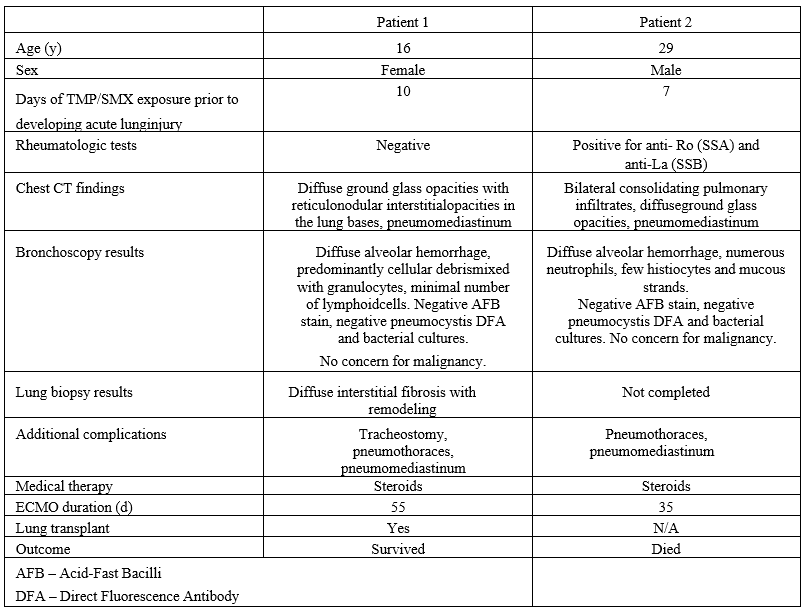
Table 1 Characteristics of patients with TMP/SMX induced lung injury
Likewise, almost all histological patterns have been associated with drug-induced lung injury, so lung biopsies are not necessary, but can be considered to rule out other causes of respiratory distress. One of our patients underwent lung biopsy prior to her lung transplant which showed diffuse interstitial fibrosis with remodeling, which can be seen in multiple pulmonary disease processes. The utility of the biopsy in this case was the exclusion of infection and malignancy. Unfortunately, our second patient was not hemodynamically stable for lung biopsy. Autopsy results for our second patient showed bilateral obliteration of alveolar airspaces from extensive hemorrhage in lungs, with fungal stain positive for Candida organism. The autopsy in our adult patient was helpful in confirming the cause of his death and highlights the importance of performing autopsies when possible in patients who expire despite extracorporeal support (8).
The variability in drug response among patients is multifactorial, including both environmental and intrinsic factors such as genetics that predispose patients to respond to drugs in a certain manner. Currently, there is no known physiologic explanation of the mechanism of lung injury caused by TMP/SMX, nor are there any identified predisposing factors that increase the risk of lung injury in patients taking TMP/SMX. One possible mechanism may be that TMP/SMX could be acting as a potential antigen in these patients, inducing an inflammatory cascade that leads to immune-mediated lung toxicity by drug-specific antibodies or drug-specific T cells (9). Both of our patients had allergic diathesis, including reactions to sulfa drugs. This may have been a predisposing factor for TMP/SMX induced lung injury (10). Additionally, the presence of DAH in both patients prior to being placed on ECMO is suggestive of a TMP/SMX associated pulmonary vasculitis as opposed to a consequence of ECMO coagulopathy. TMP/SMX has not been explicitly linked with DAH, although its association with multiple vasculitis syndromes has been described (11).
Our cases highlight the importance of health care providers having familiarity with the possible adverse effects of medications they prescribe to minimize the potential morbidity and mortality from drug- induced respiratory diseases. Patients demonstrate great variability in response to TMP/SMX ranging from non-specific constitutional symptoms, mild respiratory distress to severe hypoxic respiratory failure, which poses a major clinical diagnostic dilemma. The most common presenting symptoms appear to be dyspnea at rest with non-productive cough. A prior case series in adolescents have described presenting signs of fever, headache and pharyngitis soon after treatment of skin infections (5). Similarly, the clinical presentation in our cases included fever, shortness of breath and chest pain within two weeks of starting TMP/SMX. The duration from onset of antibiotic administration to development of symptoms appears variable as well, with prior cases (1,2,3,12) demonstrating pulmonary symptoms within 3 to 32 days of starting TMP/SMX. The presence of lung injury also does not appear to be dose dependent. Our case series adds to a limited set of cases that demonstrate failure of resolution of ARDS with conventional medical therapy, and to our knowledge, is the first report of an adult requiring venovenous extracorporeal support for severe TMP/SMX related lung injury.