Case Reports International Journal
OPEN ACCESS | Volume 3 - Issue 2 - 2025
ISSN No: 3065-6710 | Journal DOI: 10.61148/ 3065-6710/CRIJ
Said Darawshi 1*, Naim Shehadeh1,3, Michal Weiler-Sagie2,4, Afif Nakhleh 1,3
1Institute of Endocrinology, Diabetes and Metabolism, Rambam Health Care Campus, Haifa, Israel.
2Bruce Rappaport Faculty of Medicine, Technion, Haifa, Israel
3Azrieli Faculty of Medicine, Bar-Ilan University, Safed, Israel
4Department of Nuclear Medicine, Rambam Health Care Campus, Haifa, Israel
*Corresponding author: Darawshi Said, Institute of Endocrinology, Diabetes, and Metabolism, Rambam Health Care Campus, HaAliya HaShniya Street 8, Haifa 3109601, Israel.
Received Date: November 29, 2023
Accepted Date: December 08, 2023
Published Date: December 11, 2023
Citation: Darawshi S, Shehadeh N, Michal W Sagie, Nakhleh A. (2023) “Neurologic Manifestations of Tumor-Induced Osteomalacia: A Case Report.” Case Reports International Journal, 1(1); DOI: http;//doi.org/10.2023/12.1001.
Copyright: © 2023 Darawshi Said. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Tumor-induced osteomalacia (TIO) is a rare paraneoplastic syndrome that presents with progressive muscle weakness, among other symptoms. Delayed diagnosis is expected because the presentation is nonspecific, and years of debilitating symptoms may pass before a diagnosis is reached and curative treatment is applied. It is essential to increase awareness of TIO presentation, appropriate workup, and management among healthcare professionals.
We report a 28-year-old man with a 3-year history of generalized skeletal pain and progressive muscle weakness, confining him to a wheelchair. The patient had increased muscle tone with brisk deep tendon reflexes in all four limbs, positive Hoffman sign on both sides and spastic gait. Biochemical investigation revealed low serum phosphate levels and elevated alkaline phosphatase levels. Serum creatinine and calcium were normal. The bone density scan revealed severe osteoporosis. 68GA-DOTATATE positron emission tomography/computed tomography scan showed tracer uptake in a 2.5x1.6cm lesion in the right popliteal fossa and revealed multiple ribs and vertebrae fractures. Serum fibroblast growth factor 23 (FGF23) levels were elevated. The patient underwent a wide resection of the tumor. Pathology showed a mesenchymal tumor composed of spindle cells and osteoclast-like giant cells with free margins. After surgery, the patient achieved a remarkable clinical improvement with sustained normalization of serum phosphate and fibroblast growth factor 23 levels.
Background:
Tumor-induced osteomalacia (TIO) is a rare paraneoplastic syndrome characterized by renal phosphate wasting, chronic hypophosphatemia, low levels of 1,25-dihydroxyvitamin D, osteomalacia, and nonspecific symptoms including bone pain and musculoskeletal weakness. TIO is caused by mesenchymal tumors that secrete fibroblast growth factor 23 (FGF23). Locating the culprit tumor is often challenging, and complete resection is curative [1].
Case presentation:
We report a 28-year-old man presenting to our clinic with a 3-year history of generalized skeletal pain and progressive muscle weakness, confining him to a wheelchair. Physical examination revealed cervicothoracic kyphosis and tenderness in the lower limbs. Neurological examination revealed bilateral proximal weakness of upper and lower limbs, increased muscle tone with brisk deep tendon reflexes in four extremities, bilateral positive Hoffman sign, and spastic gait. There was no Babinski, and sensation was preserved.
He underwent an extensive workup, which revealed a normal NCV-EMG test, a typical head and spinal MRI, and a normal central motor conduction time in the upper and lower limbs. The cerebrospinal fluid examination was unremarkable, with negative oligoclonal bands. The neurological evaluation revealed no neurological findings that could explain the patient's symptoms and signs. The biochemical investigation showed a low serum phosphate level of 1 mg/dL (normal, 2.5 – 4.5 mg/dL) and an elevated alkaline phosphatase level of 228 U/L (normal, 30 – 120 U/L). Serum complete blood count, creatinine, urea, calcium, albumin, and 24h-urine calcium were normal. Serum 1,25-dihydroxyvitamin D was low at 10.1 pg/mL (normal, 20 – 79 pg/mL). Serum PTH level was 118 pg/mL (normal, 18 – 80 pg/mL). The percent tubular reabsorption of phosphate (%TRP) was low at 65% (reference value, 85% - 95%), indicative of renal phosphate wasting. All other blood tests were normal, including sodium, potassium, liver transaminases, B1, B6, and B12 vitamins, folic acid, TSH, ACE, VDRL/TPHA, ANA, ANCA, and complement C3 and C4. Alfacalcidol and phosphate supplements were initiated.
Dual-energy X-ray absorptiometry showed a severely reduced bone mineral density (lumbar spine Z-score of -5.5 (BMD of 0.475 g/cm2) and left femoral neck Z-score of -6.5 (BMD of 0.221 g/cm2)).
For the suspected phosphaturic mesenchymal tumor, a head-to-toe 68GA-DOTATATE positron emission tomography/computed tomography (PET/CT) scan was performed and showed tracer uptake in a 2.5x1.6cm lesion located in the right popliteal fossa (figure 1) and revealed multiple rib and vertebra fractures. Serum FGF23 levels collected from right and left median cubital and femoral veins were 1802 pg/mL, 1816 pg/mL, 2319 pg/mL, and 1806 pg/mL, respectively (normal, 23.2 - 95.4 pg/mL). Serum levels of FGF23 were measured using a fully automated chemiluminescent assay (Liaison XL, DiaSorin).
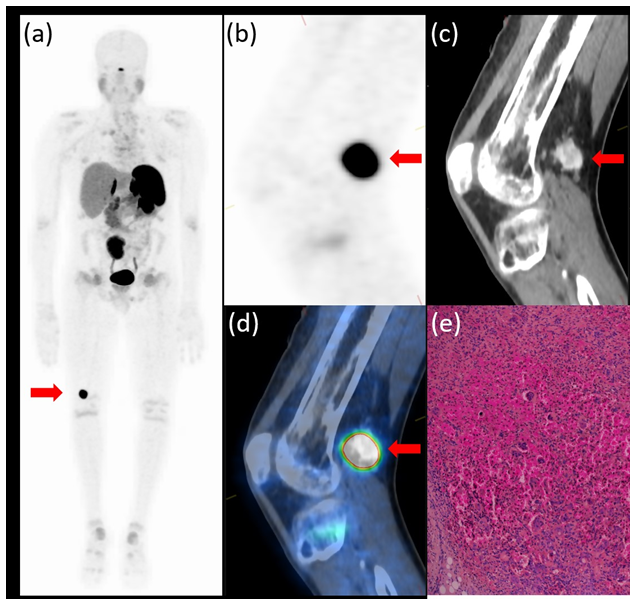
Figure 1: PET/CT 68GA-DOTATATE whole body scan maximal intensity projection (a) and images of the PET (b), CT (c), and PET/CT fusion (d) sagittal slice through the right knee showing intense uptake in an enhancing 2.5x1.6 cm well-defined lesion in the popliteal fossa (red arrow). Hematoxylin and eosin staining (x40) of a section from the lesion (e) shows a phosphaturic mesenchymal tumor composed of spindle and oval cells with an admixture of multinucleated giant cells.
The patient underwent a wide resection of the tumor. The pathology exam using Hematoxylin and Eosin staining showed a phosphaturic mesenchymal tumor made of spindle and oval cells with an admixture of multinucleated giant cells (osteoclast-like). The tumor margins were clear, and no additional immunohistochemical staining was conducted. (Figure 1e). A blood sample drawn 14 hours after surgery showed normal serum FGF23 level (52.67 pg/mL). Alfacalcidol and phosphate were stopped gradually over one week as serum phosphate levels normalized.
Over two years following the surgery, the patient experienced a remarkable clinical improvement. This included improved muscle strength, decreased bone pain, and improved ambulation, accompanied by a sustained normalization of serum phosphate (2.9 mg/dL), FGF23 (41.2 pg/mL), and alkaline phosphatase (56 U/L) levels, and a marked improvement in bone mineral density. Specifically, the lumbar spine Z-score increased to -0.8 (BMD of 1.064 g/cm2), and the left femoral neck Z-score increased to 0.5 (BMD of 1.132 g/cm2).
Discussion:
TIO diagnosis is often elusive and delayed. When suspected, laboratory tests are mandatory and should include measurement of serum phosphate, renal phosphate reabsorption, serum 1,25-dihydroxyvitamin D, and serum FGF23 levels.
FGF23 is a potent regulator of phosphate and vitamin D homeostasis. FGF23 reduces phosphate reabsorption and the production of 1,25-dihydroxyvitamin D by the kidney [2]. The primary abnormality in TIO is an increased secretion of FGF23, leading to decreased renal phosphate reabsorption and hypophosphatemia. In cells and tissues, phosphate is involved in several vital processes, including free energy transfer and tissue mineralization. Chronic hypophosphatemia results in a decreased rate of bone mineralization, which leads to osteomalacia and increases the risk for bone insufficiency fracture [1]. Chronic hypophosphatemia causes proximal myopathy, presumably by decreasing muscle ATP synthesis [3]. Interestingly, correction of serum phosphate levels in TIO often results in rapid improvement in muscle function even before the healing of osteomalacia [4]. Hypovitaminosis D and hypocalcemia in osteomalacia might also be associated with muscle weakness, albeit milder [5].
The patient described here presented with tetraparesis and underwent a thorough neurological investigation. Generally, TIO tends to give with less severe muscle weakness, but pain can be prominent [5]. However, since other causes for tetraparesis have been excluded, and the patient improved remarkably after surgery, we assume that hypophosphatemia had a promoter role in the onset of the patient's muscle weakness.
Kim et al. showed that nearly 50% of the patients with osteomalacia initially visited the neurology clinic. They found distinct characteristics of patients with osteomalacia compared to idiopathic inflammatory myopathy, including longer duration to diagnosis, frequent pain, less severe muscle weakness, and brisk deep tendon reflex [5]. However, the sample in this study was small. It included patients with osteomalacia secondary not only to hypophosphatemia but also to vitamin D deficiency, which might have presented with more subtle muscle weakness [6].
Muscle weakness has been reported in 65% of 144 TIO cases treated at a Chinese hospital [6]. TIO should be suspected in patients with osteomalacia presenting with progressive proximal muscle weakness or atrophy and bone pain. In such cases, once hypophosphatemia is detected, serum FGF23 should be measured.
Localizing phosphaturic mesenchymal tumors is challenging as they are commonly small and in unique locations are soft tissue, skin, and bone, including the upper and lower extremities and the head [7]. Whole-body, head-to-toe functional, and anatomical imaging is essential to locate the FGF23-secreting tumor since the distribution is highly variable. Because somatostatin receptors are expressed in many mesenchymal tumors of mixed connective tissue variants, an octreotide scan can help with tumor localization in about 50% of cases [1]. 18F-fluorodeoxyglucose PET/CT has good sensitivity in localizing such tumors, but it can lead to false-positive results [8]. Gallium-DOTATATE PET/CT is the emerging imaging modality for tumors producing TIO and has good sensitivity and specificity [9]. Selective venous sampling with measurement of FGF23 in conjuncture with functional modalities can confirm the tumor's location, especially in the case of multiple tumors [10].
In the patient described here, the tumor was localized to the popliteal fossa of the right knee. It is important to note that standard PET/CT imaging for common indications is usually performed from the base of the skull to the mid-thigh. The specific lesion in this patient would have been missed if the scanned area was not extended to include the knees. When referring patients to imaging for suspected TIO, a head-to-toe scan including all limbs in the studied area should be obtained.
Complete surgical resection is often curative [1]. Recurrence of TIO is rare but possible and occurs in <5% of the patients [11]. Medical therapy with phosphate supplementation and calcitriol or alfacalcidol, as well as targeted therapy such as Burosumab (a monoclonal antibody against FGF23), can be used in patients with postoperative persistent disease, patients who are not candidates for surgery, or when the culprit tumor cannot be localized [4]. Burosumab has been successfully used in a small number of patients with TIO and led to the normalization of phosphate metabolism and improvement in osteomalacia measures, fracture healing, and mobility [11].
Conclusions
TIO is potentially curable and should be suspected in the setting of hypophosphatemia and nonspecific symptoms, as early diagnosis can prevent long-term disabilities. We aim to raise awareness among clinicians about TIO, which should be suspected in the setting of chronic hypophosphatemia and proximal muscle weakness.
Declarations:
Ethics approval and consent to participate
This study was meticulous and adherent to the ethical principles outlined in the Declaration of Helsinki and with the approval of the local Helsinki committee. The safety and anonymity of all participants were paramount throughout the research process. Before enrolment, all participants provided written informed consent by signing a comprehensive consent form. All patient data are securely stored in a locked cabinet within the authors' office to safeguard their confidentiality and privacy.
Consent for publication
The patient provided written informed consent to publish their medical data, including figures. The data was carefully reviewed to ensure that it did not contain any information that could identify the patient.
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Competing Interest
The authors declare that they have no competing interests. This declaration encompasses any financial or non-financial interests that could potentially influence the research presented in this manuscript. The authors have no relevant financial relationships with commercial entities that could create a conflict of interest concerning the work described in this manuscript.
Funding Declaration:
This research received no funding from public, commercial, or not-for-profit sectors.
Author Contributions
A.N.: Conceptualization, Data curation, Formal analysis, Investigation, Interpretation, Writing - original draft
S.D.: Writing - original draft, Writing - review & editing
M.W.S.: Data curation, Formal analysis, Visualization
N.S.: Writing - review & editing
All authors approved the final version of the manuscript for submission.
Disclosure Summary: The authors declare that no conflict of interest could be perceived as prejudicing the impartiality of the research reported.