
Mahesh Kumar Acharya
College of Veterinary and Animal Science Navania, Vallabh Nagar, Udaipur, Rajasthan
*Corresponding Author: Mahesh Kumar Acharya, College of Veterinary and Animal Science Navania, Vallabh Nagar, udaipur (Rajasthan).
Received: May 05, 2021
Accepted: May 07, 2021
Published: May 17, 2021
Citation: M.K.Acharya. (2021) “Opercular Movements of Freshwater Fishes Labeo Rohita And Cirrhinus Mrigala Recorded on Kymograph Using Nitrazepam As Anaesthetic”, Journal of Agricultural Research Pesticides and Biofertilizers, 1(1); DOI:http;//doi.org/05.2021/1.1005.
Copyright: © 2021 Mahesh Kumar Acharya. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Through this research an attempt has also been made to obtain records of the opercular beats of normal and anaesthetized Labeo rohita and Cirrhinus mrigala Juveniles. These records further revealed that in general the the opercular beat, average amplitute and respiratory factor decline substantially with the increase concentration of nitrazepam and level of anaesthesia. To correlate the state of anaesthesia with the trends of opercular beats measurement of opercular beats of Labeo rohita and Cirrhinus mrigala Juveniles exposed to various doses of nitrazepam showed that anaesthetized fish always had lower opercular beat. The results further revealed that in general the opercular beats (OB), average amplitutde (AAm) and respiratory factor (RF) declined substantially with the increased levels of nitrazepam and stage of anaesthesia. The efficacy of the use of nitrazepam in inducing light and deep sedation in the two experimental fishes is summarized below (Table 1.1)
|
S.No. |
Fish |
Anaesthetic |
Stage of anaesthetic |
Average |
|
|
OB |
AAm |
||||
|
1. |
Labeo rohita |
Nitrazepam dose range (0.5 and 5.0 mg/l) |
Light sedation |
9.64 |
5.1 |
|
Deep Sedation |
9.37 |
2.38 |
|||
|
2. |
Cirrhinus mrigala |
Nitrazepam dose range (1.0 and 5.0 mg/l) |
Light sedation |
12.37 |
4.35 |
|
Deep Sedation |
11.87 |
2.09 |
|||
Introduction:
The tranquillizers are also called as psychotropic drugs or alternatively as 'ataractics' such as Reserpine, Phenotheazine derivatives, Benzodizepine and Meta cloprimides. A large number of solid and liquid anaesthetics are available for use in fishery science. However, water soluble anaesthetics are more suitable for fish. Further, the chemical nature and properties of drugs affects fish behaviour and their physiology. According, the dose applied may vary with anaesthetics as well as the species of the fish to be anaesthetized.
Anaesthetics could be important mean for handling fish during operation such as artificial spawning, marking, weighing, measuring, transportation, finclipping, tagging, photography and such as other operative procedures. Anaesthetizing agents are becoming important in aquaculture for producing a wide range of effect varying from mild sedation to complete loss of equilibrium and in sensitivity. The use of anaesthetics in fishery is so widespread that every year millions of fish is anaesthetized for the purpose of surgery, handling, photography, transportation and operative activities. It has been consistent endeavor of scientists to search for an ideal ichthyo-anaesthetic agent for application in various fishery procedures. Various sedatives and hypnotic drugs could be categorized into four major groups on the on the basis of chemical characteristics i.e. Ethane derivatives, baribiturates, non-barbiturate hyponotics and sedative and tranqullizers. Ether is the oldest anesthetic agent known general anaesthesia in medical and veterinary sciences. Morever, MS-222 has been adjudged as the most suitable for use as Ichthyo anaesthetic agent. Using MS-222 even a shark weighing a tone was successfully.
All the sedative drugs do not have same effect on the animal exposed to such drugs and therefore, the response of these drugs is varied and depend on age, body weight emotional state, metabolic disease, previous history of medicine with narcotics etc. US Food and Drug Administration (FDA) has set certain requirements for registration of fish anaesthetics. So far only tricaine sulphate (MS-222) and carbondioxide (CO2) are registered by FDA for use of trot fish (Schnick et al., 1979). Fish being a palatable commodity, the selection and use of any anaesthetics must conform to the public health safety standards. In general, anaesthetics with proven public health safety are chosen as fish anaesthetics. The anaesthetics of most common fisheries use is quinaldine and MS-222. Quinaldine in used for photographic work but also wide acceptance in fishery work (Gilderhus et al., 1977).
Materials and methods:
For recording opercular beats of Labeo rohita and Cirrhinus mrigala (Juveniles) Kymographic equipment was employed. In this study anaesthetic nitrazepam was used due to its short induction and recovery time. Varius four doses were used in this experimental i.e. 0.5, 1.0, 3.0 and 5.0 mg/l for Labeo rohita 1.0, 2.0, 3.0 and 5.0 for Cirrhinus mrigala.
(Figure 1.1) depicts the kymographic equipment and the lever attached with holding clamp. Juveniles of Labeo rohita and Cirrhinus mrigala were fixed in the holding clmap without putting much stress on the body of fish. Starling heart lever was adapted for recording opercular beats. A glazed paper with a fine layer of carbon soot, was fixed to the kymographic drum and the speed of the drum was kept at 1.25 mm/sec. opercular beats were recorded after the fish was fastened in the clamp.
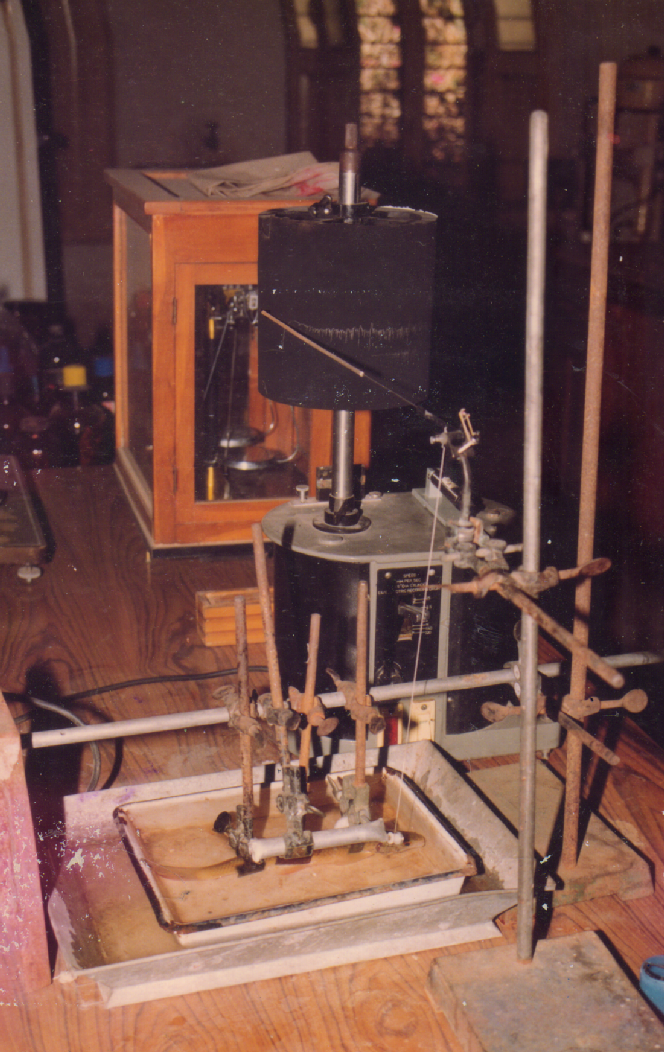
Figure 1.1: Kymographic equipment setup with lever and fish holding device for recording the opercular beats of fish.
After acclimatizing the fish laboratory condition for 4-5 hours, opercular beats of normal fish were recorded. To anaesthetize the clamped fish, a calculated volume of drug nitrazepam was added to the water in tray. For taking the opercular records the fishes were kept immersed in water or anaesthetic media in a tray having three litres of water oxygenated with air diffuser stone. Thereafter opercular beats were recorded at intervals ranging between 1-15 minutes.
For assessing stage of anaesthesia another fish was kept in a tray with same strength of solution of drug. Recovery from anaesthesia was recorded at a dose of 5.0 mg/l in Labeo rohita and Cirrhinus mrigala. The recording made on paper were processed using mixture of Damer resin and sprit.
On the basis of such records opercular beats / 10 sec. (OB), average amplitudes in mm (AAm) at a particular time were tabulated. Similarly, respiratory factor (RF) was calculated using following relationship.
Average amplitude (mm) of Opercular beats (AAm)
RF = ---------------------------------------------------------------- x 100
Number of opercular beats in 10 sec. (OB)
The interpretation of recording for opercular beats are based on the opening or closure of gill flaps. A downwards wave indicates closing of gill flaps whereas upwards wave is due to opening of the same. Sometimes on abrupt increase in the amplitude of opercular beats is also evident due to occasional coughing the experimental fish.
Results and discussion:
With the use of especially adapted kymographic equipment the opercular beats for two species of experiment fishes Labeo rohita and Cirrhinus mrigala were recorded. Before recording opercular beats the experimental fish were acclimatized for orbit a week in the circular plastic container.
The lowest concentration of nitrazepam i.e. 0.5 mg/l tried on Labeo rohita and Cirrhinus mrigala yearlings induced light sedation after 15 minutes (Table 1.2 and Plate 1.1).
In the case the records of opercular beats were made at an interval of 15 minutes upto 75 minutes after post anaesthesia. For each observation records OB were taken for a period of 10 second. In the normal fish records of OB were 13.50/10 sec. control. In anaesthetized state the OB reduced to 9.5/10 sec. (after 60 minutes) and increase slightly thereafter to 10.00/10 sec. at deep sedation (state 2). In this case average amplitude (AAm) in the normal fish was 6-12 mm. During anaesthesia the AAM varies between minimum of 1.87 (75 min.) to as high as 5.02 (15 min).
The respiratory factor of Labeo rohita yearlings exposed to 0.5 mg/l nirazepam declines as compared to normal fish. RF in normal fish was 45.33 whereas in anaesthetized fish in varied between 18.70 (75 min) to the highest of 39.20 (45 min). Table 1.2 further reveals that various in RF didnot show regularity. Nevertheless, at light sedation the RF never feel below 25.90.
The percent reduction in RF maximum (58.74) at the stage of deep sedation after 75 minutes (Table 1.2). During light sedation maintained from 15 to 60 minutes percent reduction in RF fluctuated between a lowest of 13.52 to as high as 42.86.
|
S.No. |
Parameters |
Time (minutes) at which opercular beats were recorded |
|||||
|
0.00 |
15.0 |
30.0 |
45.0 |
60.0 |
75.0 |
||
|
1. |
Opercular amplitude (AAm) in mm |
6.12 |
5.02 |
2.72 |
3.92 |
2.50 |
1.87 |
|
2. |
Opercular beats (OB) per 10 sec. |
13.50 |
13.00 |
10.50 |
1000 |
9.50 |
10.00 |
|
3. |
Respiratory factor (RF) |
45.33 |
38.61 |
25.90 |
39.20 |
26.31 |
18.70 |
|
4. |
Per cent reduction in RF |
- |
14.82 |
42.86 |
13.52 |
41.95 |
58.74 |
|
5. |
Stage of control fish |
Normal |
LS |
LS |
LS |
LS |
DS |
Table 1.2: Observation on opercular movement of Labeo rohita recorded on Kymograph using 0.5 mg/l nitrazepam.
|
S.No. |
Parameters |
Time (minutes) at which opercular beats were recorded |
|||||
|
0.00 |
10.0 |
15.0 |
25.0 |
35.0 |
45.0 |
||
|
1. |
Opercular amplitude (AAm) in mm |
7.45 |
6.87 |
5.05 |
3.45 |
2.35 |
1.57 |
|
2. |
Opercular beats (OB) per 10 sec. |
8.00 |
7.50 |
7.50 |
7.00 |
8.00 |
8.00 |
|
3. |
Respiratory factor (RF) |
93.12 |
91.60 |
67.3 |
49.28 |
29.37 |
19.62 |
|
4. |
Per cent reduction in RF |
- |
1.63 |
27.69 |
47.07 |
68.46 |
79.93 |
|
5. |
Stage of control fish |
Normal |
LS |
LS |
LS |
DS |
DS |
Table 1.3: Observation on opercular movement of Labeo rohita recorded on Kymograph using 1.0 mg/l nitrazepam.
|
S.No. |
Parameters |
Time (minutes) at which opercular beats were recorded |
|||||
|
0.00 |
5.0 |
10.0 |
15.0 |
20.0 |
25.0 |
||
|
1. |
Opercular amplitude (AAm) in mm |
8.12 |
8.02 |
4.87 |
2.52 |
2.02 |
1.10 |
|
2. |
Opercular beats (OB) per 10 sec. |
11.50 |
12.00 |
10.00 |
8.00 |
6.50 |
6.00 |
|
3. |
Respiratory factor (RF) |
70.60 |
66.83 |
48.70 |
31.50 |
31.07 |
18.33 |
|
4. |
Per cent reduction in RF |
- |
5.33 |
31.01 |
55.38 |
55.99 |
74.03 |
|
5. |
Stage of control fish |
Normal |
LS |
LS |
DS |
PLE |
TLE |
Table 1.4: Observation on opercular movement of Labeo rohita recorded on Kymograph using 3.0 mg/l nitrazepam
|
S.No. |
Parameters |
Time (minutes) at which opercular beats were recorded |
|||||
|
0.00 |
3.0 |
7.0 |
11.0 |
15.0 |
20.0 |
||
|
1. |
Opercular amplitude (AAm) in mm |
9.05 |
6.45 |
4.30 |
3.10 |
1.97 |
0.92 |
|
2. |
Opercular beats (OB) per 10 sec. |
10.00 |
8.00 |
11.00 |
11.50 |
12.50 |
12.00 |
|
3. |
Respiratory factor (RF) |
90.50 |
80.62 |
39.09 |
26.95 |
15.76 |
7.66 |
|
4. |
Per cent reduction in RF |
- |
10.91 |
56.80 |
70.22 |
82.58 |
91.53 |
|
5. |
Stage of control fish |
Normal |
LS |
LS |
DS |
PLE |
TLE |
Table 1.5: Observation on opercular movement of Labeo rohita recorded on Kymograph using 5.0 mg/l nitrazepam.
|
S.No. |
Parameters |
Time (minutes) at which opercular beats were recorded (Recovery phase) |
||||
|
5.0 |
15.0 |
30.0 |
40.0 |
60.0 |
||
|
1. |
Opercular amplitude (AAm) in mm |
1.50 |
1.55 |
2.87 |
4.25 |
4.97 |
|
2. |
Opercular beats (OB) per 10 sec. |
11.50 |
11.00 |
9.50 |
10.50 |
11.00 |
|
3. |
Respiratory factor (RF) |
13.04 |
14.09 |
30.21 |
40.47 |
45.18 |
|
4. |
Per cent reduction in RF |
85.59 |
84.43 |
66.61 |
55.28 |
50.07 |
|
5. |
Stage of control fish |
Toward normalization in freshwater |
Toward normalization |
Toward normalization |
Toward normalization |
Almost normal |
Table 1.6: Observation on opercular movement of recovering Labeo rohita recorded on Kymograph with 5.0 mg/l nitrazepam.
|
S.No. |
Stages of fish |
OG/10 Sec. |
AAm (mm) |
RF |
% decline in RF |
|
1. |
Normal |
10.75 |
7.68 |
74.88 |
- |
|
2. |
Light sedation (LS) |
9.64 |
5.11 |
54.87 |
26.44 |
|
3. |
Deep sedation (DS) |
9.37 |
2.36 |
25.41 |
64.50 |
|
4. |
Partial loss of equilibrium (PLE) |
9.50 |
10.99 |
23.41 |
69.28 |
|
5. |
Total loss of equilibrium (TLE) |
9.00 |
1.01 |
12.99 |
82.78 |
Table 1.7: OB, AAm & RF of Labeo rohita Juveniles at variuos anaesthetics stages using nitrazepam.
|
S. No. |
Parameters |
Time (minutes) at which opercular beats were recorded |
|||||||
|
0.00 |
4.0 |
8.0 |
12.0 |
16.0 |
20.0 |
30.0 |
40.0 |
||
|
1. |
Opercular amplitude (AAm) in mm |
5.40 |
3.97 |
3.05 |
4.40 |
3.00 |
1.75 |
2.15 |
1.87 |
|
2. |
Opercular beats (OB) per 10 sec. |
12.00 |
10.00 |
9.00 |
9.50 |
8.50 |
10.00 |
10.00 |
10.00 |
|
3. |
Respiratory factor (RF) |
45.00 |
39.70 |
33.88 |
46.31 |
35.29 |
17.50 |
25.50 |
18.70 |
|
4. |
Per cent reduction in RF |
- |
11.77 |
24.71 |
2.91* |
21.57 |
61.11 |
52.22 |
58.44 |
|
5. |
Stage of control fish |
Normal |
LS |
LS |
LS |
LS |
DS |
DS |
DS |
* Increase in RF
Table 1.8: Observation on opercular movements of C. mrigala recorded on Kymograph using 1.0 mg/l nitrazepam.
|
S. No. |
Parameters |
Time (minutes) at which opercular beats were recorded |
|||||
|
0.00 |
10.00 |
20.00 |
30.00 |
40.00 |
50.00 |
||
|
1. |
Opercular amplitude (AAm) in mm |
12.15 |
5.67 |
5.75 |
3.17 |
1.25 |
1.17 |
|
2. |
Opercular beats (OB) per 10 sec. |
13.00 |
13.50 |
13.50 |
12.00 |
11.50 |
12.00 |
|
3. |
Respiratory factor (RF) |
93.46 |
42.00 |
42.59 |
26.41 |
10.86 |
9.75 |
|
4. |
Per cent reduction in RF |
- |
55.06 |
54.42 |
71.74 |
88.38 |
89.56 |
|
5. |
Stage of control fish |
Normal |
LS |
LS |
DS |
PLE |
PLE |
Table 1.9: Observation on opercular movements of C. mrigala recorded on Kymograph using 2.0 mg/l nitrazepam.
|
S. No. |
Parameters |
Time (minutes) at which opercular beats were recorded |
||||||
|
0.00 |
5.0 |
10.0 |
15.0 |
20.0 |
30.0 |
40.0 |
||
|
1. |
Opercular amplitude (AAm) in mm |
7.10 |
6.05 |
2.62 |
1.12 |
1.29 |
2.27 |
1.10 |
|
2. |
Opercular beats (OB) per 10 sec. |
12.00 |
12.50 |
10.50 |
11.50 |
10.50 |
9.00 |
9.50 |
|
3. |
Respiratory factor (RF) |
59.16 |
48.40 |
24.95 |
9.73 |
12.28 |
25.22 |
11.57 |
|
4. |
Per cent reduction in RF |
- |
18.18 |
57.82 |
83.55 |
79.24 |
57.36 |
80.44 |
|
5. |
Stage of control fish |
Normal |
LS |
DS |
DS |
PLE |
PLE |
TLE |
Table 1.10: Observation on opercular movements of C. mrigala recorded on Kymograph using 3.0 mg/l nitrazepam.
|
S. No. |
Parameters |
Time (minutes) at which opercular beats were recorded |
|||||
|
0.00 |
4.0 |
8.0 |
12.0 |
20.0 |
30.0 |
||
|
1. |
Opercular amplitude (AAm) in mm |
7.35 |
2.27 |
1.87 |
1.40 |
1.29 |
0.57 |
|
2. |
Opercular beats (OB) per 10 sec. |
15.00 |
14.00 |
14.50 |
14.50 |
14.00 |
15.50 |
|
3. |
Respiratory factor (RF) |
49.00 |
16.21 |
12.89 |
9.65 |
9.21 |
3.67 |
|
4. |
Per cent reduction in RF |
- |
66.91 |
73.69 |
80.30 |
81.20 |
92.51 |
|
5. |
Stage of control fish |
Normal |
LS |
LS |
DS |
PLE |
TLE |
Table 1.11: Observation on opercular movements of C. mrigala recorded on Kymograph using 5.0 mg/l nitrazepam.
|
S. No. |
Parameters |
Time (minutes) at which opercular beats were recorded (Recovery phase) |
|||
|
5.0 |
20.0 |
40.0 |
60.0 |
||
|
1. |
Opercular amplitude (AAm) in mm |
1.52 |
5.27 |
6.35 |
7.25 |
|
2. |
Opercular beats (OB) per 10 sec. |
9.50 |
14.00 |
13.00 |
14.50 |
|
3. |
Respiratory factor (RF) |
16.00 |
37.64 |
48.84 |
50.00 |
|
4. |
Per cent reduction in RF |
67.34 |
23.18 |
0.326 |
2.04* |
|
5. |
Stage of control fish |
Toward normalization in freshwater |
Toward normalization |
Toward normalization |
Almost normal |
* - Increase in RF
Table 1.12: Observation on opercular movements of C. mrigala recorded on Kymograph with 5.0 mg/l nitrazepam
|
S.No. |
Stages of fish |
OB/10 sec. |
AAm (mm) |
RF |
% decline in RF |
|
1. |
Normal |
13.00 |
8.00 |
61.65 |
- |
|
2. |
Light sedation (LS) |
12.37 |
4.35 |
36.00 |
41.39 |
|
3. |
Deep Sedation (DS) |
11.87 |
2.09 |
18.15 |
69.99 |
|
4. |
Partial loss of equilibrium (PLE) |
11.83 |
1.42 |
12.75 |
79.49 |
|
5. |
Total loss of equilibrium (TLE) |
12.50 |
0.83 |
7.62 |
86.47 |
Table 1.13: OB, AAm & RF of C. mrigala Juvenils at various anaesthetic stages using nitrazepam.
In the case of 1.0 mg/l nitrazepam, the records of OB of normal fish were 8.00/10 sec. In anaesthetized stage the OB reduced to 7.00/10 sec. (after 25 minutes) and increased slightly thereafter 8.00 / 10 sec. after 35 and 45 minutes at deep sedation. In this case the average amplitude (AAm) in the normal fish was 7.45 mm. During anaesthesia the AAm fluctuated between a minimum of 7.00 (25 min) to maximum of 8.00 (35 and 45 min). The RF of Labeo rohita yearlings exposed to 1.0 mg/l nitrazepam declined as compared to normal fish, RF in normal fish was 93.12 whereas in anesthetized fish fluctuated between lowest of 19.62 (45 min.) to the highest of 91.80 (10 min.) (Table 1.3 and Plate 1.2).
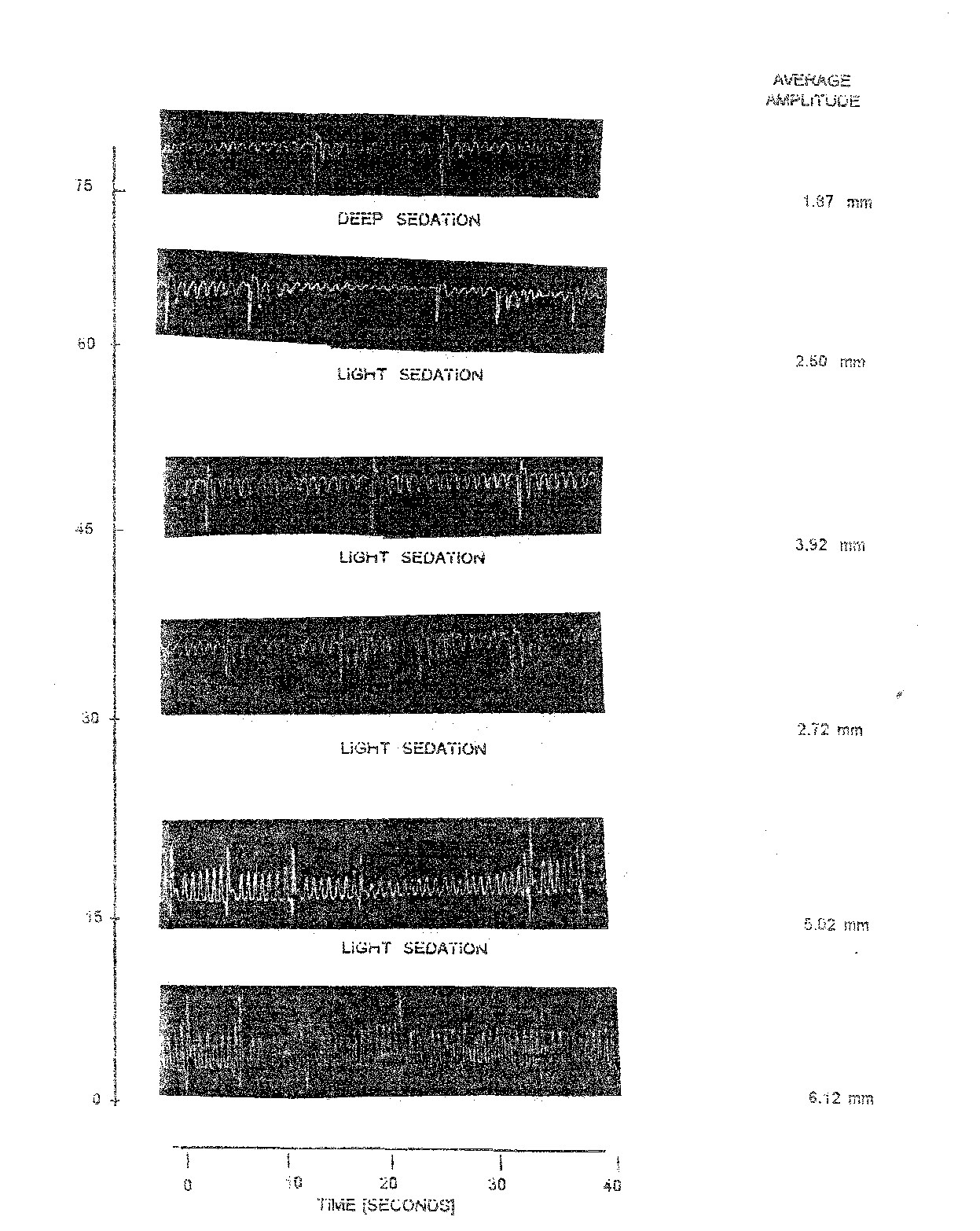
Plate 1.1: Opercular beats of normal and nitrazepam @ 0.5 mg/l treated fish [l. Rohita] at varying time intervals
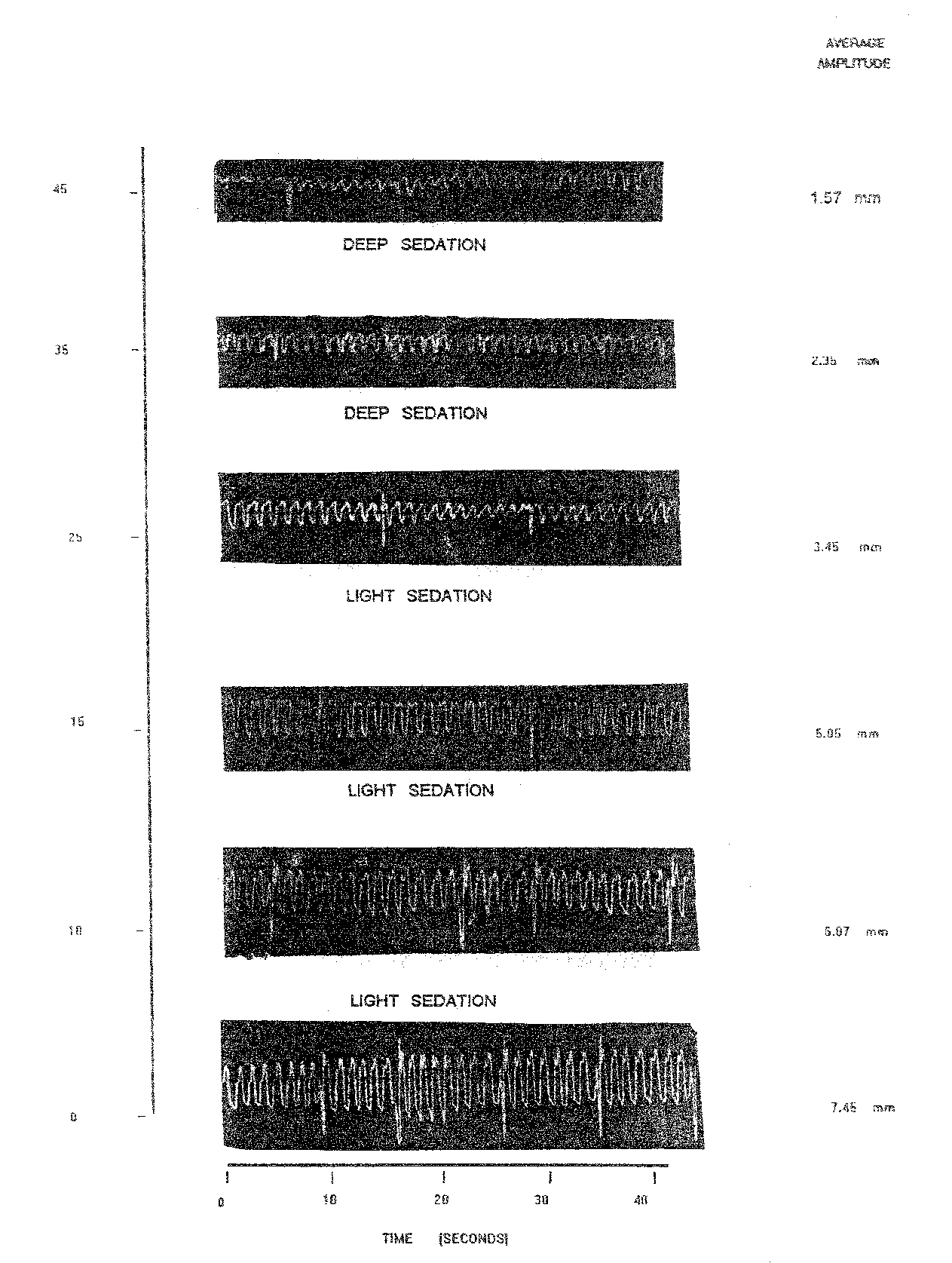
Plate 1.2: opercular beats of normal and nitrazepam @ 1.0 mg/l treated fish [l. rohita] at varying time intervals
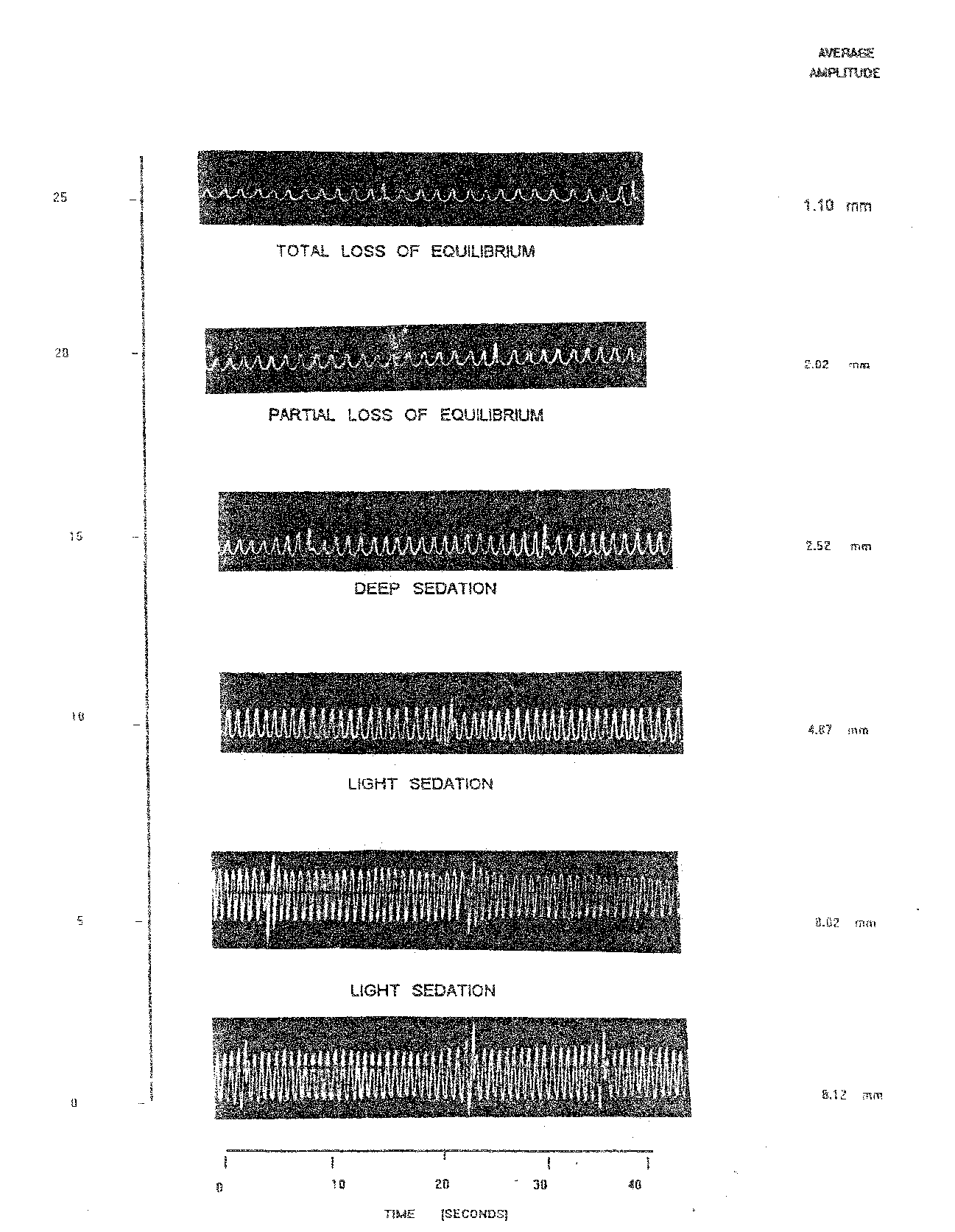
Plate 1.3: opercular beats of normal and nitrazepam @ 3.0 mg/l treated fish [l. Rohita ] at varying time intervals
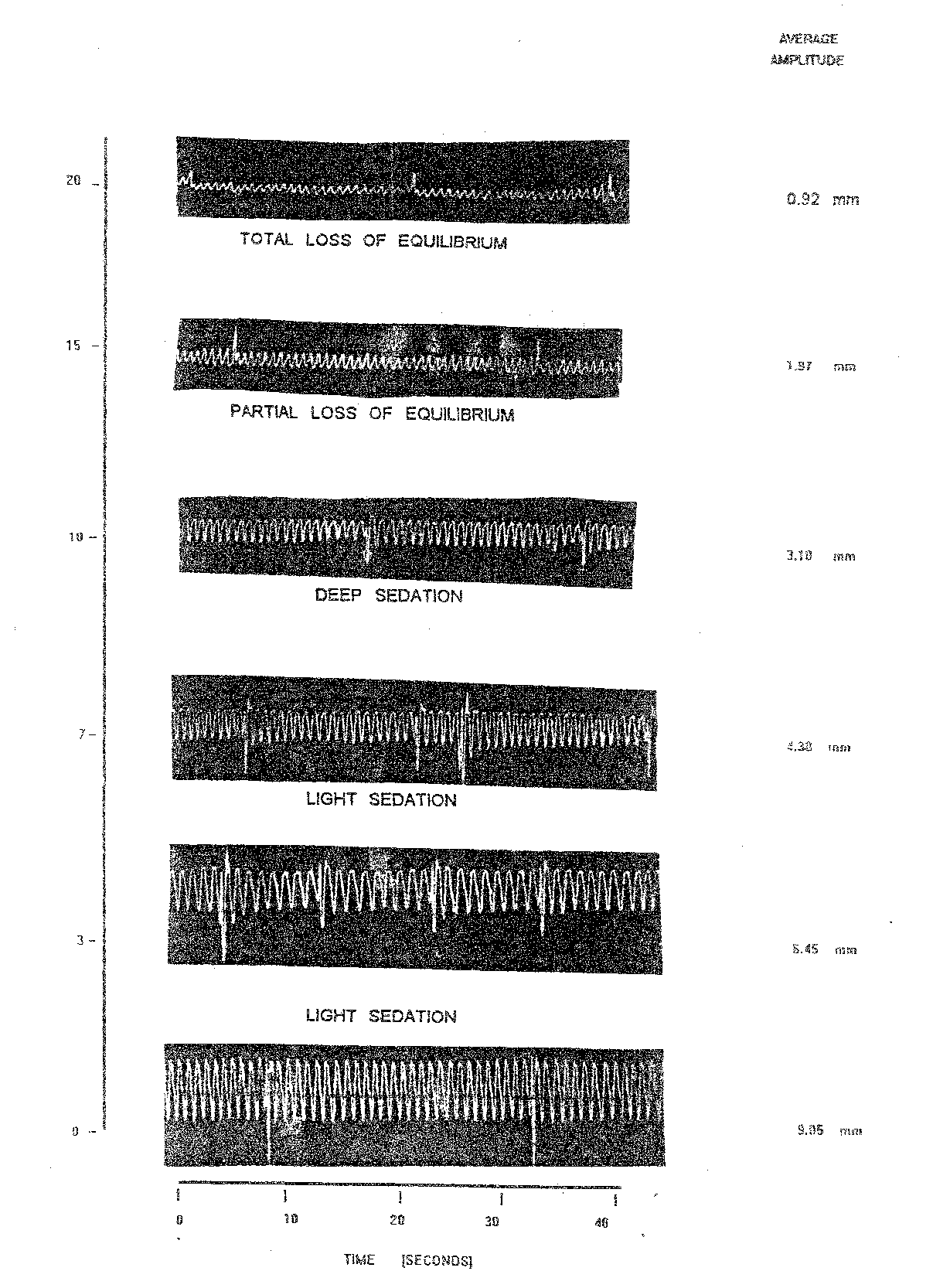
Plate 1.4: opercular beats of normal and nitrazepam @ 5.0 mg/l treated fish [l. Rohita ] at varying time intervals
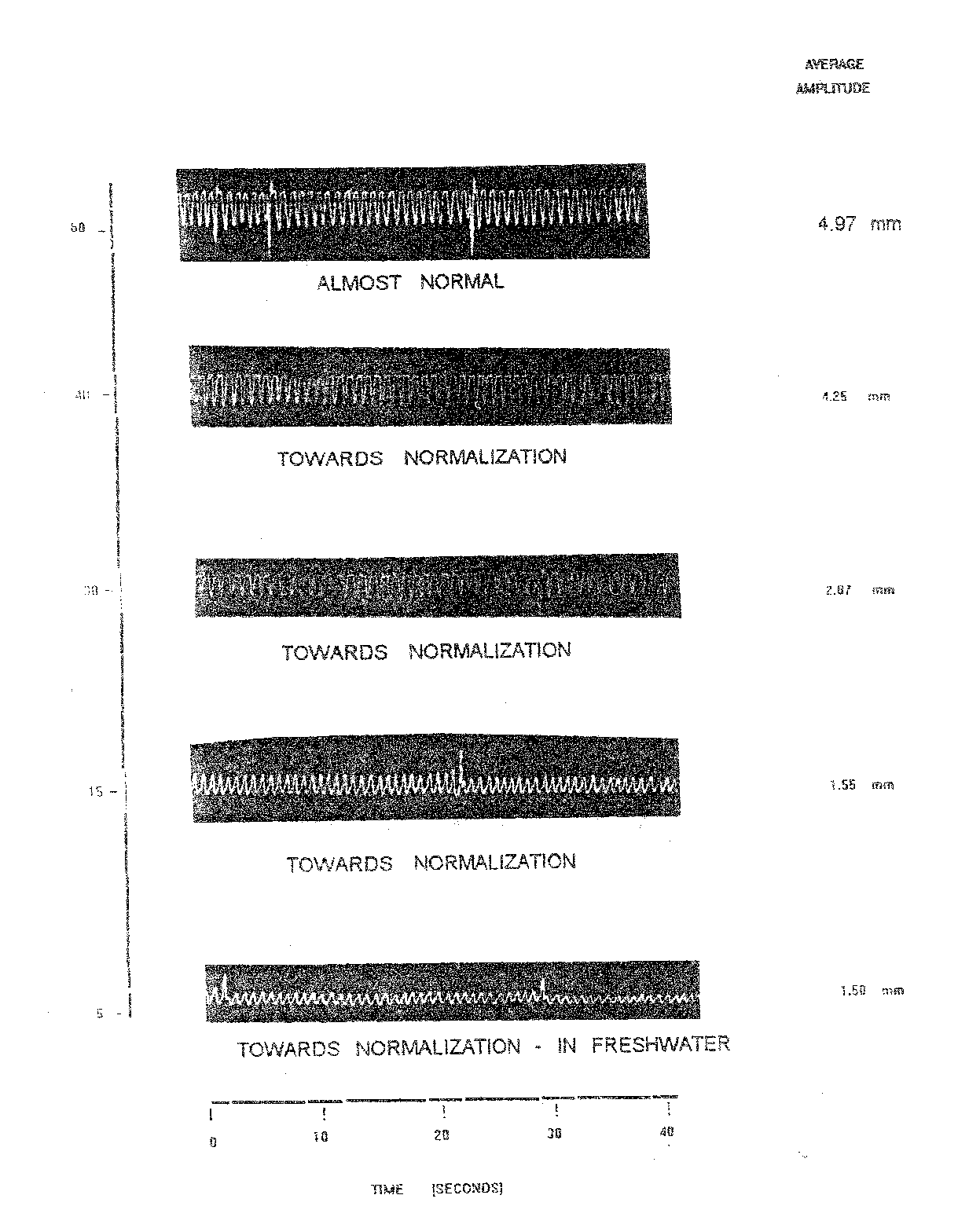
Plate 1.5: opercular beats of recovering fish [l. Rohita] treated with 5.0 mg/l nitrazepam.
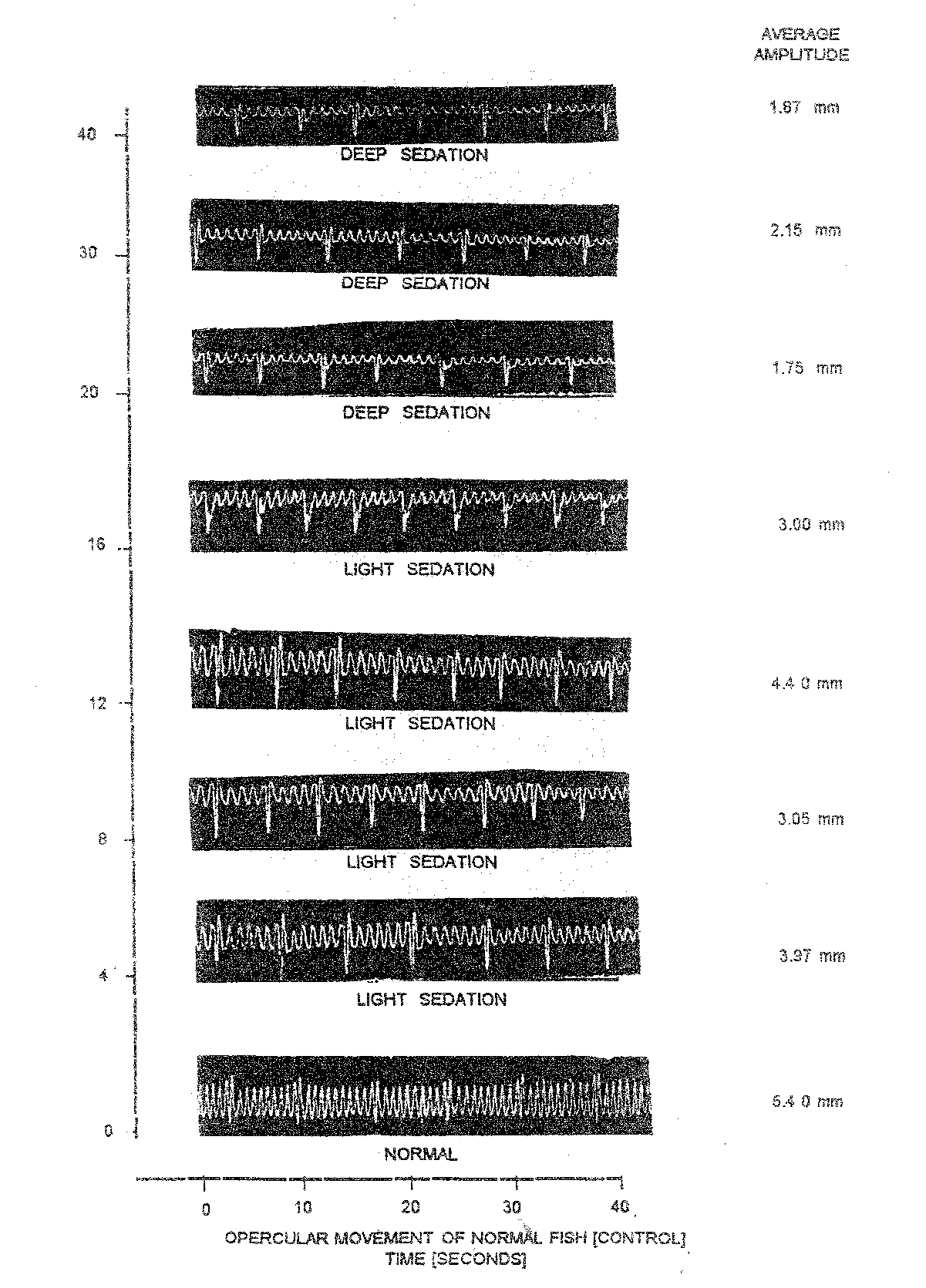
Plate 1.6: opercular beats of normal and nitrazepam @ 1.0 mg/l treated fish [c. Mrigala] at varying time intervals.
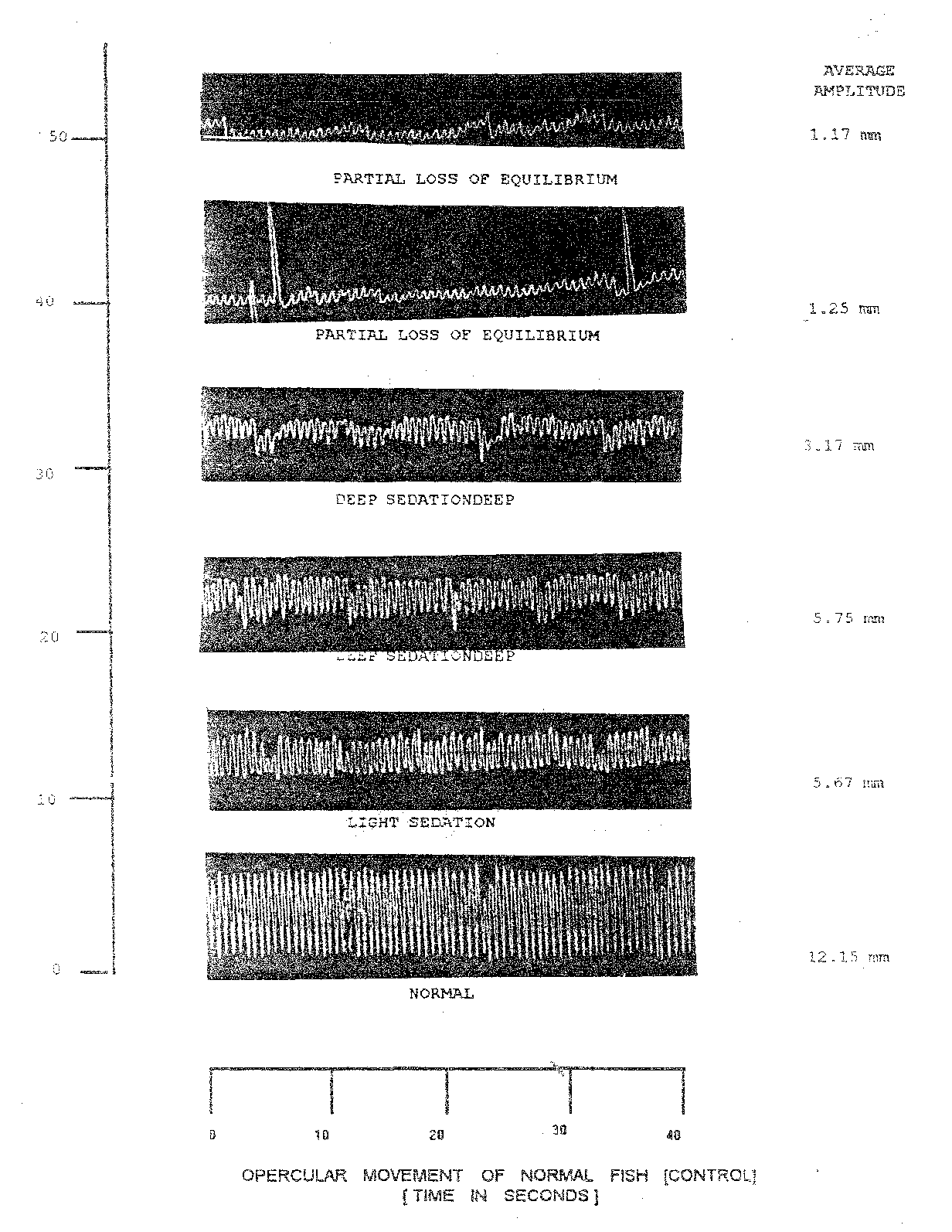
Plate 1.7: opercular beats of normal and nitrazepam @ 2.0 mg/l treated fish [c. Mrigala] at varying time intervals
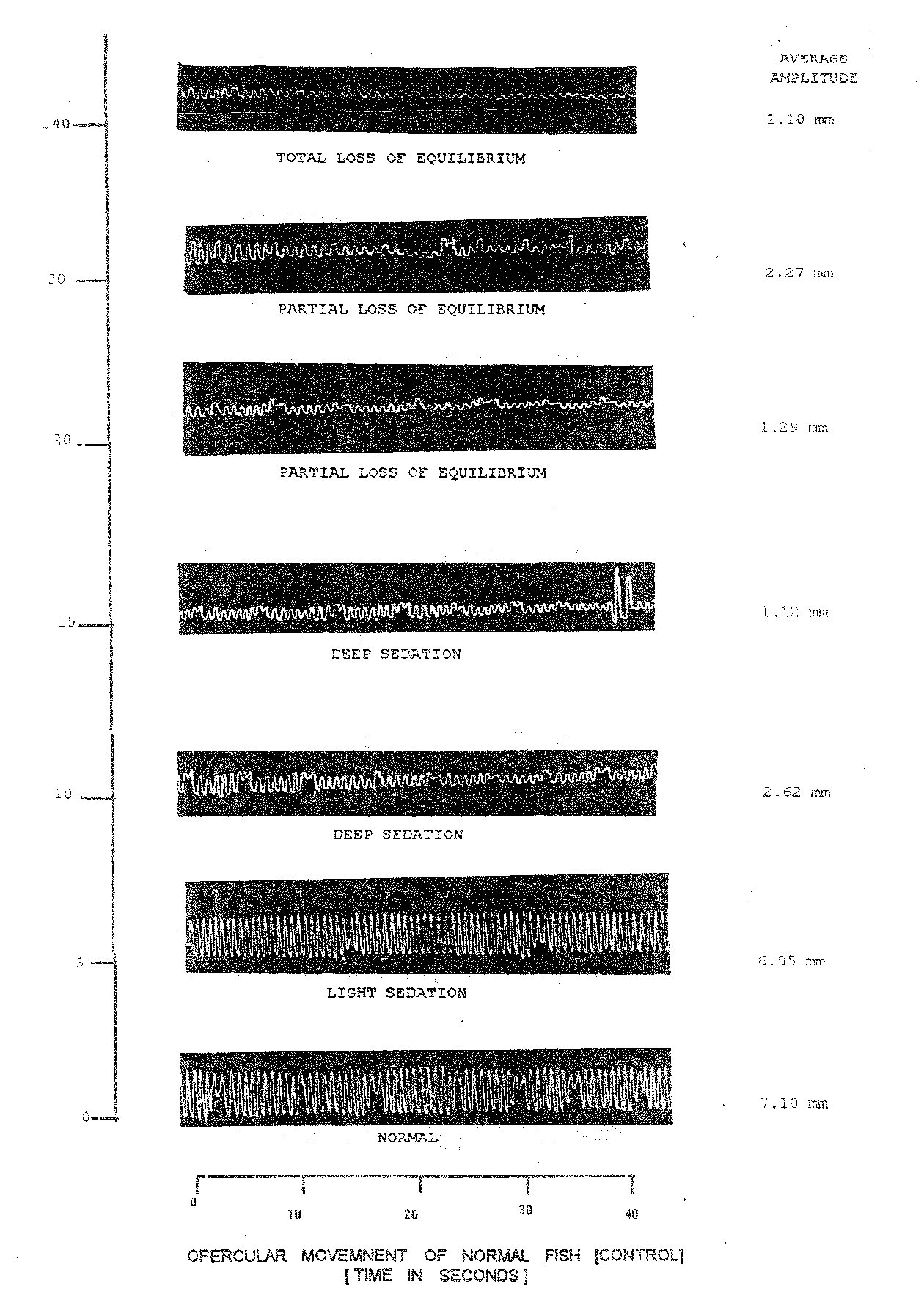
Plate 1.8: opercular beats of normal and nitrazepam @ 3.0 mg/l treated fish [c. Mrigala ] at varying time intervals
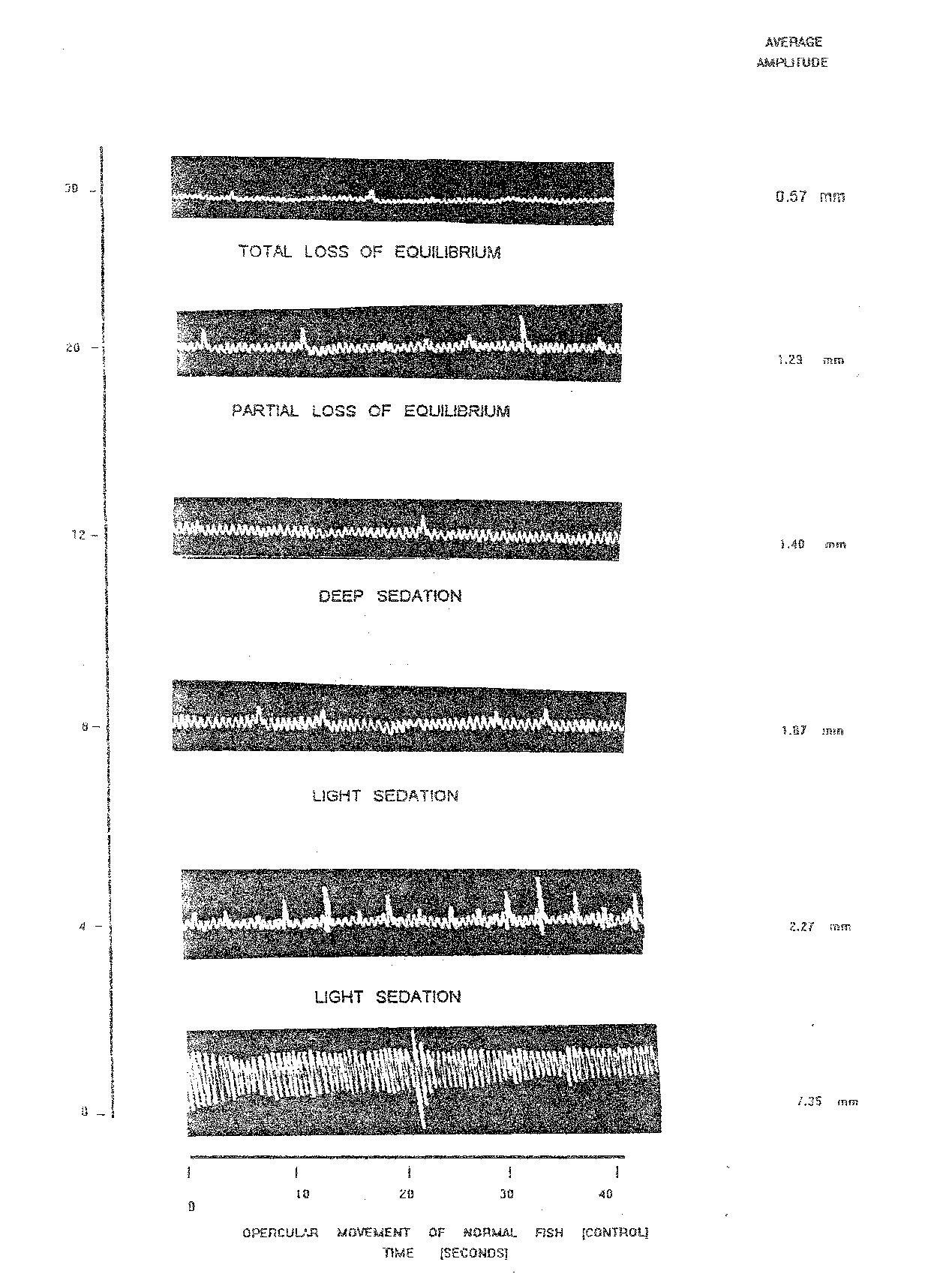
Plate 1.9: opercular beats of normal and nitrazepam @ 5.0 mg/l treated fish [c. Mrigala ] at varying time intervals
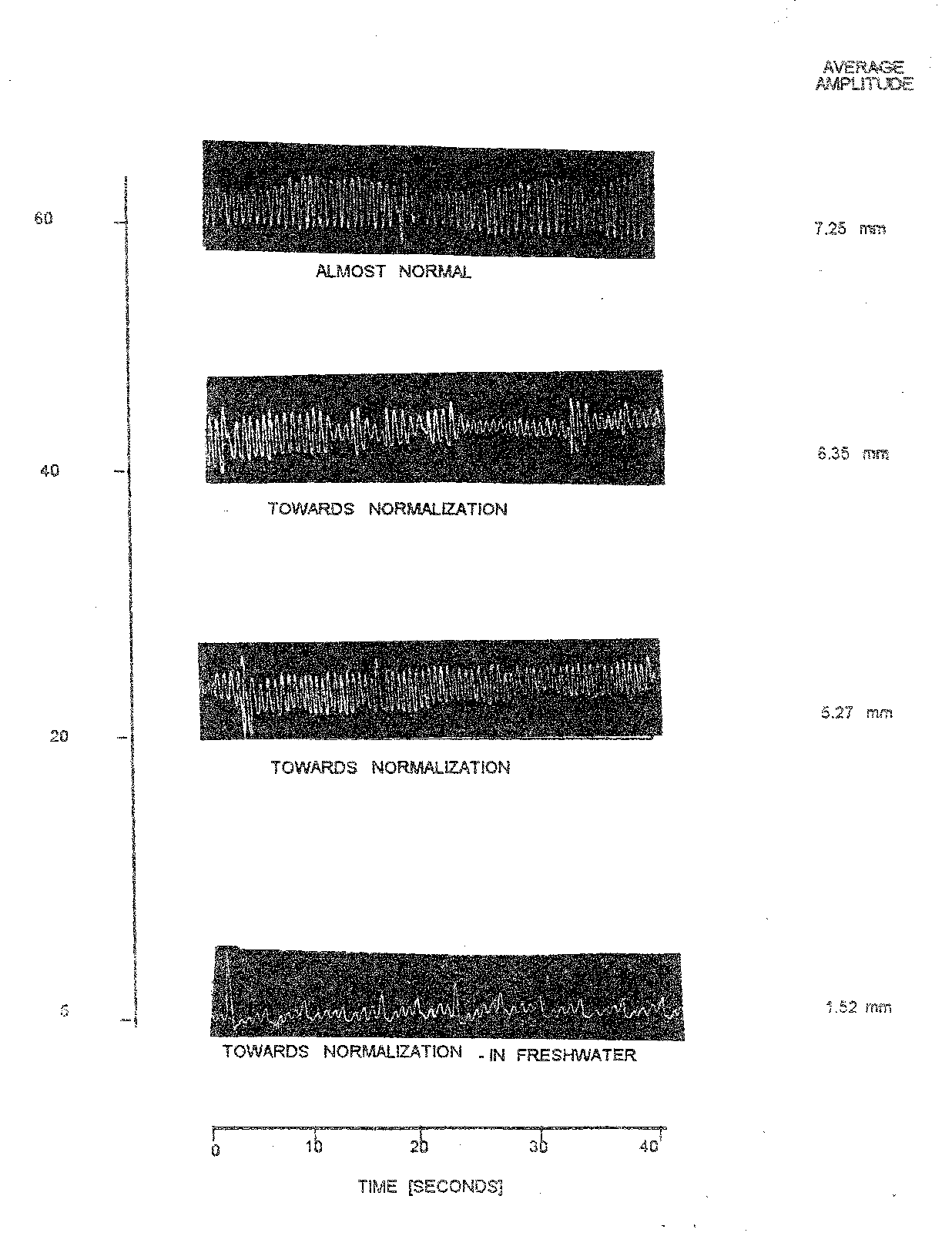
Plate 1.10: opercular beats of recovering fish [c. Mrigala ] treated with 5.0 mg/l nitrazepam.
The percent reduction in RF was highest (79.83) at the stage of deep sedation after 45 minutes (Table 1.3). During light sedation which was maintained from 10 to 25 minutes percent reduction in RF varied between a minimum of 1.63 to maximum of 47.07.
The third dose of nitrazepam @ 3.0 mg/l (Table 1.4 and Plate 1.3) exhibited wide variation in opercular movements. In the normal fish the OB was 11.50 / 10 sec. Increase in OB was 12.00/10 sec at the stage of light sedation after 5 minutes. Which reduced progressively with the induction of anaesthesia to minimum of 6.00/10 sec. at 25 minutes. In this case the AAm in the normal fish was 8.12 mm. During anaesthesia the AAm fluctuated between minimum of 1.10 (25 min.) to the maximum of 8.02 mm (5 min.).
The respiratory factor of Labeo rohita yearlings exposed to 3.0 mg/l nitrazepam declined as compared to normal fish. RF in normal fish was 70.60 whereas in anaesthetized fish in varied between lowest 18.33 (25 min.) to the highest of 68.83 (5 min.). The percent reduction in RF was maximum 74.03 (25 min.) at the stage of total loss of equilibrium. Light sedation maintained during 5 to 10 minutes percent reduction in RF varied between 5.33 to 31.01.
Result of the highest concentration of nitrazepam 5.0 mg/l tried on Labeo rohita yearlings are presented in (Table 1.5, plate 1.4). In this case of the OB of normal fish were 10.0/10 sec. which increased initially to 12.50/10 sec. at 15 minutes. After 20 minutes, the OB was slightly reduced (12.00/10 sec.). When fish attained total loss of equilibrium (state 4). In this case the AAm in the normal fish was 9.05 mm. During anaesthesia the AAm recorded (Table 1.5) were 3.10, 1.97 and 0.92 mm at 11, 15 and 20 minutes, respectively. Showing the stage of deep sedation, partial loss of equilibrium and total loss of equilibrium. The RF of control fish was 90.50 which was reduced upto 7.66 (at 20 min.). The reduction of RF was maximum at the stage of total loss of equilibrium after 20 minutes i.e. 91.53 percent decline (Table 1.5). The declines at light and deep sedation stage were 10.91 and 70.22 percent respectively (Table 1.5).
Table 1.6 and Plate 1.5 depict than during recovery at 5 minutes the fish exhibited abnormal opercular movements without any regularity in OB and amplitude. In this stage the OB and AAm were 11.50/10 sec. and 1.50 mm respectively. The fish was almost at normal stage after 60 minutes of change in fresh water. Normal respiratory activity in further evident by a similar pattern of increased regular AAm and slight increase in OB. At this time recorded OB was 11.00/10 sec. and AAm was 4.97 mm and RF of 45.18 percent reducing in RF was 50.07.
Considering all the treatments with the use of nitrazepam the variation in OB of normal fish were from 8.00 (Table 1.3) to 13.50 (Table 1.2) whereas AAm shwed variation range of 6.12 (Table 1.2) to 9.05 (Table 1.5). Similarly, the RF in normal fish fluctuated from a minimum of 45.33 (Table 1.2) to maximum of 93.12 (Table 1.3).
Compared to normal fish, the anaesthetized fish always had lower OB which varied between 6.00 (Table 1.4) to 13.0 (Table 1.2). The AAm in the anaesthetized fish in different doses showed lowest values of 0.92 mm (Table 1.5) to the highest of 8.02 (Table 1.4). Likewise, the RF values in anaesthetized fish were always lower than the normal fish and exhibited variation from the lowest of 7.66 (Table 1.5) to highest of 91.60 (Table 1.3) as evident from (Table 1.7). The percentage decline in RF in anesthetized fish was minimum of 26.44 during light sedation at different doses. Such percentage decline in RF further growth with the advancement of anaesthesia to attain the highest percentage decline of 82.78 when the fish achieved total loss of equilibrium.
The dose of nitrazepam tried on Cirrhinus mrigala yearlings was 1.0 mg/l (Table 1.8and Plate 1.6). In this case the recording of opercular beats were made at time intervals of 4, 8, 12, 16, 20, 30- and 40-minutes post anaesthesia. In anaesthetized fish, the opercular beats declined to 8.50/10 sec. of the stage of light sedation (18 minutes) but increased thereafter to 10.00/sec. at light to deep sedation after 16 minutes. During anaesthesia the average amplitude verified between the lowest of 1.75 (20 min.) to as higher as 4.40 (12 min.) respiratory factor in anaesthetized fish it varied between 17.50 (20 min.) Table 1.8 further reveals that variation in respiratory factor did not show regularity. The maximum per cent reduction in respiratory factor was 61.11 (20 min.) at the stage of deep sedation (Table 1.8). During light sedation maintained from 4 to 16 minutes per cent reduction in respiratory factor varied between from 4 to 16 minutes per cent reduction in respiratory factor varied between a maximum of 2.91 to as high as 21.57.
In the Table 1.9 and Plate 1.7 the results for a dose of 2.0 mg/l nitrazepam, the anaesthetized stage of the opercular beats reduced to 11.50/10 sec. (at 40 min.) and increased slightly thereafter to 12.00/10 sec when the fish undergone partial loss of equilibrium. During anaesthesia in average amplitude fluctuated between a minimum of 1.17 (50 min.) to as higher as 5.75 (20 min.). The corresponding respiratory factor values showed a marked decline from 42.00 to 9.75 in anaesthetized fish as compared to the normal fish exhibiting respiratory factor values of 93.46 (Table 1.9). The percentage decline in respiratory factor values at light and deep sedation stages were 55.06 and 71.74 respectively.
The third dose of Nitrazepam @ 3.0 mg/l (Table 1.10 and Plate 1.8) tried on Cirrhinus mrigala yearlings exhibited widevariations in opercular movements. In anaesthetized stage the opercular beats in Cirrhinus mrigala fingerling reduced to 9.00/10 sec (after 30 min.) and increased slightly thereafter to 9.5/10 sec. when fish attained total loss of equilibrium. The average amplitude was reduced a minimum of 1.10 AAm at total loss of equilibrium after completion of 40 minutes. The respiratory factor of normal fish was observed to be 59.16 which showed substantial reduction upto 11.57 (at 40.0 min.). The reduction in respiratory factor was thereafter 40 minutes i.e. 80.44 per cent decline (Table 1.10). The variation in declines at light and deep sedation were 18.18 and 83.55 per cent, respectively.
In the (Table 1.11 and Plate 1.9) results of the highest concentration of nitrazepam @ 5.0 mg/l are show in anaesthetized state the opercular beats reduced to 14.00/10 sec. (after 20 min.) and increased thereafter to 15.50/10 sec. at total loss of equilibrium. The average amplitude of anaesthetized fish fluctuated between lowest of 0.57 (30 min.) to as high as 2.27 (4.0 min.). The respiratory factor of Cirrhinus mrigala yearlings exposed to 5.0 mg/l nitrazepam in anaesthetized fish in fluctuated between minimum of 3.67 (30 min.) to the maximum of 16.21 (4 min.). The per cent reduction in respiratory factor was maximum (92.51) at the stage of total loss of equilibrium after 30 minutes. During light sedation, which was maintained from 4 to 8 minutes per cent reduction in respiratory factor values between 66.91 to 73.69.
As shown in (Table 10.12 and Plate 1.10) the anaesthetized fish did not recover to normal condition when the anaesthetic medium in the tray was replaced by normal fresh water. At 60 minutes the opercular beats further increased 14.50/10 sec. to with corresponding average amplitude of 7.25 mm and respiratory factor value of 50.00 considering all the treatment with the use of nitrazepam the variation in opercular beats of normal fish from (Table 1.8 to Table 1.10) to 15.00 (Table 1.8). Likewise, average amplitude showed variation range between 5.40 (Table 1.8) to 12.5 (Table 1.9). Similarly, the RF in normal fish in different doses between the lowest of 45.00 (Table 1.8) to highest of 93.48 (Table 1.9).
From these data it is further clear that comprised to normal fish the anesthetized fish always had lower opercular beats which fluctuated between 8.50 (Table 1.8) to 15.30 (Table 1.11). The average amplitude in fish anaesthetized with different doses of nitrazepam showed lowest values of 0.57 (Table 1.11) to the highest of 6.05 (Table 1.10). The average of respiratory factor in anesthetized fish was lowest 7.62 for TLE while it was 36 for LS stage considering all the doses of nitrazepam together. As shown (Table 1.13) indicate that the percentage decline in respiratory factor in anaesthetized fish was lowest of 41.39 during light sedation. Such percentage decline in RF further grown with the advancement of anaesthesia and attained maximum percentage decline of 86.47 when the fish achieved total loss of equilibrium.
Various method for recording opercular beats in fish and other animals are on record (Good man and Weinberger, 1971, Spoor and Drumm and 1972). Opercular beats may be measured by direct observations (Belding, 1929; Ellis, 1937 and Jones 1947 by mean of electrodes or transducers attached to the fish (Halsh band and Halsh band, 1968 and Sutterlin, 1969). The electrode chamber method described by Spoor et al. (1971) in a reliable technique for measuring opercular and locomotor movements of most free-swimming fishes. According to Rommel (1973) recording fish heart and opercular beats directly from the water in possible within a limited range of water respectively. Sharma (1972) reported opercular beats of Labeo rohita Juveniles exposed to quinaldine to correlate the state of anaesthesia with opercular beats.
In the present observations compared to normal fish, the anesthetized fish always had lower opercular beats. The AAm in the anesthetized fish in different doses showed lowest Values at this stage of total loss of equilibrium (Table 1.6 and Table 1.11) and the highest at the stage of light sedation among the anesthetized fish. Further, the respiratory factor (RF) values in anesthetized fish were always lower than the normal fish. Such percentage decline in respiratory factors further attained greater magnitude of decline with the advancement of anaesthesia, when the fish achieved total loss of equilibrium (Table 1.6 and Table 1.11). As shown in (Table 1.7 and Table 1.13) the average calculated for all the doses of nitrazepam indicate that the percentage decline in respiratory factor in anaesthetized fish was lower during light sedation and highest with total loss of equilibrium. The OB measured for the fish under recovery phase indicated that after a time lapse of 60 minutes in Labeo rohita attained almost normally (Table 1.6 and Table 1.12). They besides, visual observation as regards fish behaviors opercular beats caused serve as good tool for assessing the state of anesthesia (Sharma 1992). Respiratory frequency, amplitude of breathing and ventilation volume of fish have been measured by a number of workers (Hughes, 1964 and 1971, Randall, 1970 and Shelton, 1970). In this connection, the present observations on opercular beats for two Indian major Carps bear significance.