Agricultural Research Pesticides and Biofertilizers
OPEN ACCESS | Volume 2 - Issue 1 - 2025
ISSN No: 2994-0109 | Journal DOI: 10.61148/2994-0109/ARPB
Oluwatoyin Sunday Osekita1, Abiola Toyin Ajayi2*, Alaba Emmanuel Gbadamosi3, Alexander Jibayo Akinwekomi4 and Olanrewaju Taiwo Fagade5
Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba-Akoko, Nigeria.
*Corresponding authors: Abiola Toyin Ajayi, Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba-Akoko, Nigeria.
Received Date: June 01, 2022
Accepted Date: September 30, 2022
Published Date: November 01, 2022
Citation: Oluwatoyin Sunday Osekita, Abiola Toyin Ajayi, Alaba Emmanuel Gbadamosi, Alexander Jibayo Akinwekomi and Olanrewaju Taiwo Fagade. (2022) “Genotypic Variability and Plant Character Correlation Among the Wheat (triticum aestivum l.) Genotypes”, 4(2); DOI:http;//doi.org/10.2022/1.1079
Copyright: © 2022 Abiola Toyin Ajayi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This study was done during the rainy season of 2015/2016 for the estimation of genotypic variability and plant character correlations in ten genotypes of wheat. The experiment was laid out in a randomized complete block design (RCBD) consisting of three replicates in the field. The quantitative characters measured were: plant height, spike length, number of tiller per meter square (m2), days to 50% heading, number of days to maturity, number of spikes per meter square (m2), number of grain per 10 spikes, the weight of 10 spikes, 1000 grain weight (g) and grain yield in g/net plot. Grain yield had the highest estimate of the phenotypic coefficient of variance PCV (95.65) and genotypic coefficient of variation GCV (92.31). Heritability was highest for days to 50% heading (99.53%) and least for spike length (5.56%). The genetic gain was highest for grain yield/net plot (18.77%) and lowest for spike length (0.37%). Grain yield significantly correlated with days to 50% heading (0.63), the number of grains/spike (0.47), and the weight of spike (0.66). Therefore, these yield component characters showed wide genetic variation and can be explored for further improvement programs for grain yield in wheat breeding.
wheat; variability; character; genotypes; yield
Introduction:
Wheat is an annual grass with a height in the range of half to one and a quarter meters, it has a long stalk that terminates in a tightly formed cluster of plump kernels enclosed by a beard of bristly spikes (Smith, 2010). It is grown all over the world for its high nutrient content and useful grain, as one of the top three most produced crops, along with maize and rice. Wheat compared to other cereal and legumes is cultivated over a wide range of climatic conditions and an understanding of its growth is of great value to plant breeders (Osekita and Ajayi, 2013). Genetic variability, the basic ingredients of breeding could be achieved through the hybridization of diversified genetic material as well as the introduction of exotic germplasm. Several workers have found out the significance of variation in test materials hence creating an avenue for improvement on the existing genotypes. Singh et al. (2006), Ghimirary et al. (2000) and Shazly et al. (2000) found significant genetic variability and higher heritability estimates with greater values of genetic advance for the number of spikes, number of grains per spike, 100 seed weight, plant height, and grain yield. Aafia et al. (2000), however, observed low to high estimates of heritability and genetic advance for these quantitative traits except plant height. Moghaddam et al. (2006) and Shafiq et al. (2006) studied 53 pure lines of bread wheat and reported significant genotypic differences for most of the yield traits with high heritability estimates. The observations of Singh and Chowdhury (1985) and Deshmukh et al. (2006) showed a high value of the phenotypic and genotypic coefficient of variability, heritability, and genetic advance in plant biomass, grain yield, tillers per plant, plant height, and the number of grains per spike assuring scope of yield improvement through selection. Ajmal et al. (2009); Akhter et al. (2008) and Ali et al. (2008) observed significant genotypic differences in the traits including plant height, number of productive tillers per plant, number of spikelets per spike, spike length, number of grains per spike, fertility percentage, 1000 grain weight, and grain yield. They also reported higher estimates of GCV, PCV, and heritability for the number of productive tillers per plant, number of grains per spike, and grain yield per plant. Akcura (2009) noted low heritability estimates ranging from 12.9 to 50.0 percent. He observed the highest expected genetic advance for grain yield (8.35%). The degree of heritability indicates the reliability through which the genotypes are recognized by their phenotypic expression Chandrababu and Sharma. (1999). Pramoda and Gangaprasad (2007) had classified the heritability values into various categories from low (< 40%) to very high (>80%) estimates. Eid (2009) observed low and medium heritability for different yield traits in wheat under rainfed conditions. Subhani et al. (2011) and Shabbir et al. (2012) found highly significant differences in days to heading, plant height, tillers per square meter, 1000-grain weight, and grain yield in some local exotic (CIMMYT) germplasm under drought stress and irrigated conditions. Baranwal et al. (2012) noted wide genetic variation among wheat genotypes for days to heading, plant height, tillers, and grains per spike. The development of high-yielding varieties requires the knowledge of existing genetic variability and offers better scope for selection Osekita et al. (2015). Hence, the study was conducted to estimate genetic variability and plant character correlations among wheat genotypes in rain-fed conditions.
Materials and Methods:
A field experiment was conducted with ten wheat genotypes during the year 2015/2016 cropping season at the breeding plots of the Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba-Akoko, southwest, Nigeria. The ten genotypes of wheat were obtained from the Lake Chad Research Institute and are presented in Table 1
|
S/N |
Variety Name |
|
1 |
PASTOR-2/KATTILA-13//HAMAN-5 |
|
2 |
Somama-9/ICAADA-SRAL-2 |
|
3 |
ATTILA-7CHECK |
|
4 |
REYNA 28 |
|
5 |
ATTILA 50Y//ATTILA/BCN/3/STARTMUSK-3 |
|
6 |
ATTILA-7/KAT |
|
7 |
KAUZ’S/SERI/3/TEVEE’S//CROW/VEE’S |
|
8 |
ATTILA-GAN-ATTILA |
|
9 |
HUBARA-3*2/SHUHA-4 |
|
10 |
SERI.IB |
Table 1: List of wheat genotypes
The experimental field was ploughed, harrowed and top dressed with NPK 15, 15, 15. The wheat genotypes were planted directly in the open field in a plot laid out in randomized complete block design (RCBD) with three replications. Each genotype was planted in a row to row and plant to plant distance of 25cm, 5 weeks after planting, ten representative plants for each genotype in each replication were randomly selected to record observations on ten quantitative traits viz, plant height, number of tillers, number of days to spike emergence, number of days to maturity, spike length, 1000 grain weight, number of grains per spike, grain yield/net plot.
Statistical Analysis:
The data collected on each character were subjected to statistical analysis. Analysis of variance (ANOVA) was done following the method of Panse and Sukhatme (1962) and Osekita and Olorunfemi (2014). Genetic variance (Vg), phenotypic variance (Vp), genetic coefficient of variance (GCV) and phenotypic coefficient of variance (PCV), heritability in the broad sense (h2), and genetic advance as percentage of mean were estimated.
Genetic variance (Vg) = MsTrts- Ms (Error)R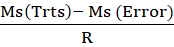
Phenotypic variance (Vp) = Vg + Ms (Error)
Genetic coefficient of variance (GCV) = Vgx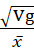 × 100
× 100
Phenotypic co-efficient of variance (PCV) = Vpx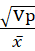 × 100
× 100
Heritability in broad sense (h2B) = VgVp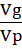 × 100
× 100
Genetic advance (Ga) = h2× K × Vp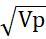
Where K is equal to selection constant at 5% = 2.06
Genetic advance as percent of mean = h2× K × Vpx 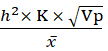 × 100
× 100
x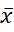 = mean of the population.
= mean of the population.
Results:
The mean sum of squares due to various sources of variation for measured quantitative characters such as Plant height, 50% heading in days, physiological maturity, number of tillers per m2, number of spikes per m2, spike length, number of grain per ten (10) spikes, grain yield, 1000 grain weight and weight of ten 10 spikes are presented in Table 2. The analysis of variance showed that almost all the parameters measured were significant at a 0.05% probability level except for grain yield which is not significant.
The coefficient of variation (CV) for all the characters ranged from 6.02% for the number of days to physiological maturity to 106.0% for grain yield/ net plot. Others have CVs below 30.0% except for characters such as number of spikes per meter square (30.06%), number of grains per 10 spikes (36.27%), and weight of 10 spikes (85.39%).
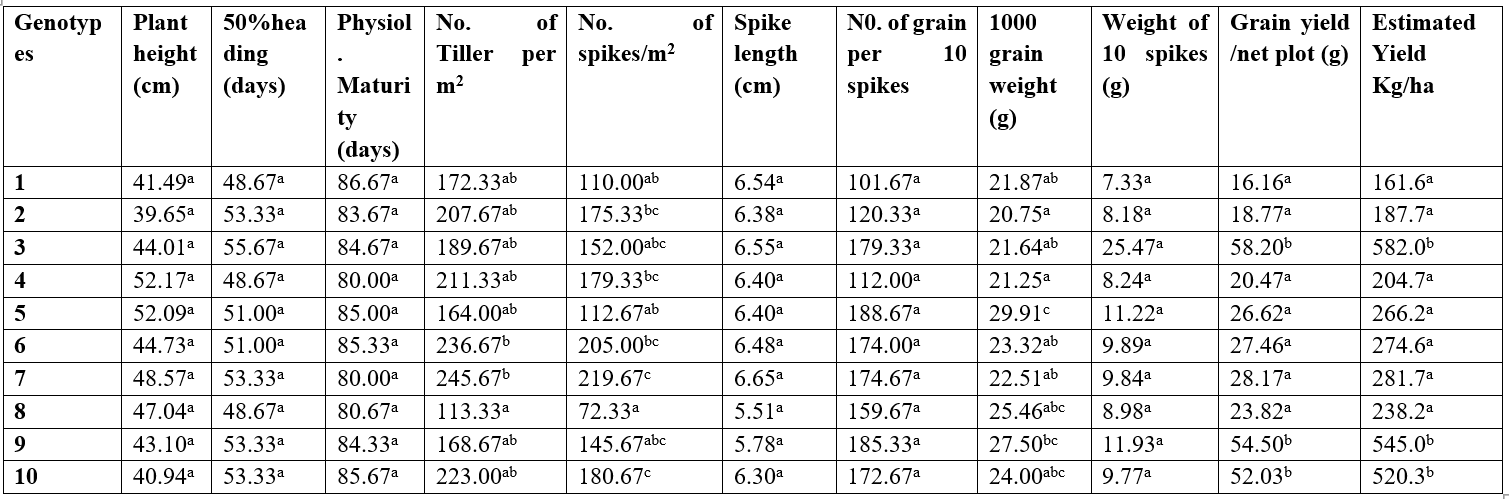
The mean performance of ten quantitative characters in wheat is presented in Table 3. Plant height indicated no significant difference among the genotypes, with Somama-9/ICAADA-SRAL-2 having the shortest height of 39.65cm and the tallest is REYNA 28 with 52.17cm. The number of days to 50% heading showed no significant difference among genotypes PASTOR-2/KATTILA-13//HAMAN-5, REYNA 28, and ATTILA-GAN-ATTILA having the lowest mean number of days to heading 48.67 days and the highest is found in ATTILA-7CHECK 55.67 days. Physiological maturity also revealed no significant difference among the genotypes, with KAUZ’S/SERI/3/TEVEE’S//CROW/VEE’S maturing early in about 80.00 days and PASTOR-2/KATTILA-13//HAMAN-5 matures late in 86.67days. In tiller per m², the result showed that almost all the varieties were not different from each other, with variety 8 (ATTILA-GAN-ATTILA) having the lowest number of tillers at 113.33±37.8, and variety 7 (KAUZ’S/SERI/3/TEVEE’S//CROW/VEE’S) having the highest number of tillers per m2 (245.67±52.3). The number of spikes/m² also indicated no significant difference among the genotypes, with variety 8 having the lowest number of spikes (72.33±7.33) and the highest number of spikes in variety 7 (219.67±39.48). Spike length indicated no significant difference among the genotypes, with variety 8 having the shortest spike of 5.51.33±0.56cm and the longest in variety 7 (6.65±0.03cm). The number of grains per 10 spike also indicated no significant difference among the genotypes, with variety 1 (PASTOR-2/KATTILA-13//HAMAN-5) having the lowest mean value (101.67±23.99) and the highest in variety 5 (ATTILA 50Y//ATTILA/BCN/3/STARTMUSK-3) (188.67±7.13). Grain yield per net plot also indicated no significant difference among the genotypes, with variety 1 having the lowest mean value of 16.16±8.82g/net plot and the highest in variety 3 (ATTILA-7CHECK) 58.20±32.96g/net plot. In 1000 grain weight, the result showed that almost all the varieties were not different from each other, with variety 4 (REYNA 28) having the lowest mean value of 21.25±1.37 g and variety 5 having the heaviest weight of 29.91±2.10 g. The weight of 10 spikes indicated no significant difference among the genotypes, with variety 1 having the lowest weight of 7.33±1.57g and the heaviest weight in variety 3 (25.47±16.71 g).
Estimates of genetic parameters consisting of genotypic coefficient of variance (GCV), phenotypic coefficient of variance (PCV), heritability (h2), Genetic advance, and Genetic advance as percent of mean were presented in Table 4. The phenotypic coefficient of variation (PCV) is higher than the genotypic coefficient of variation (GCV) in almost all the traits measured but in the number of days to 50% heading GCV is higher at 7.43% while PCV was 7.00%. Heritability estimates in a broad sense were found to be very high (i.e. > 70) for the number of days to 50% heading (99.53%), the weight of 10 spikes (94.18%), grain yield/net plot (93.15%), days to physiological maturity (91.91%) and the number of tillers per m2 (75.12%). While plant height (59.15%), number of spikes per m2 (34.27%), 1000 grain weight (33.95%), and number of grains per 10 spikes (29.12%) had moderate heritability. Very low heritability was noticed in spike length (5.56%). The genetic advance was very low in spike length 0.37% but very high in grain yield/net plot (18.77%). Genetic advance as percent of mean ranged from very low 2.26% in the number of grains per 10 spikes to very high 160.60% in weight of 10 spikes.
Correlation coefficient analyses of the characters measured are presented in Table 5. The number of spikes per m2 had a very high significant positive correlation (0.91) with the number of tillers per m2 and a highly significant correlation with the number of days to 50% heading (0.40). Grain yield/net plot had a very high significant positive correlation (0.66) with the weight of 10 spikes, number of days to 50% heading (0.63), and number of grains per 10 spikes (0.47). The weight of 10 spikes also correlates positively with the number of days to 50% heading (0.38). The number of grains per 10 spikes had a significant positive significant correlation with the number of days to 50% heading (0.39). Days to physiological maturity showed a positive significant correlation (0.37) with days to 50% heading whereas it had a negative significant correlation with plant height (-0.41). A highly significant negative correlation was noticed between plant height and number of days to 50% heading (0.47).
Discussion:
Wheat plays a major role in the sustainable food security of the country. Weeds and stress conditions especially during early crop growth are the two major constraints limiting its yield. However, the use of cultivars with early maturity and seedling vigor facilitates good crop establishment and better competitive ability with weeds; this is an important strategy for enhancing and stabilizing the productivity of wheat. Often high seedling vigor is associated with bold and heavy seeds, which are not preferred by consumers as compared to slender grains. Hence, there is an urgent need to assess variability and interrelationship between seed and seedling traits as breeding strategies for quality grain features in wheat cultivars. However, lack or inadequacy of water at the early stage of wheat growth hinders or delays heading and according to observations noticed during the experiment, a partial initiation of the spike which does not fully emerge is likely not to have seeds inside the spike due to partial maturity. The best wheat is produced in areas favoured with cool, moist weather during the major portion of the growing period followed by dry, warm weather to enable the grain to ripen properly. The optimum temperature range for ideal germination of wheat seed is 20-250C. But the seeds can germinate in the temperature range of 30-350C which is the temperature of the test environment. Rains just after sowing hamper germination and encourage seedling blight. Therefore areas with a warm and damp climate are not suited for wheat growing, this is a major reason why the productivity level of wheat growing in the tropical rainforest, particularly during the wet season will not lead to increased yield consequent upon the temperature gradient of the zone.
The highest output obtainable was 582 kg/ha compared to cool, moist, and dry climate which favors high wheat production of about 1368 kg/ha according to the World bank record of 2011 in Northeastern Nigeria. During the heading and flowering stages, excessively high or low temperatures and drought are harmful and therefore not suited for wheat growing. Also, cloudy weather with high humidity and low temperature is conducive to rust attack. An optimum average temperature of 14-150C is required at the time of ripening. During grain filling and development, a temperature below 250C is required above which the grain weight is depressed. When temperatures are high, too much energy is lost through the process of transpiration by the plants, and the reduced residual energy results in poorer grain formation and lower yields according to Shabbir et al. (2012). Excessive rainfall during maturity increases fungal infection but less or moderate rainfall during this period reduces fungal infection such as glume blotch and scab commonly known as fusarium head blight which can also be caused by corn stalk on the soil surface and over fertilizer application should be avoided in other to keep away from powdery mildew as earlier reported by Robert (2002). Lodging is most common after heading when growth has been overstimulated by excess nitrogen fertilizer or moisture, high temperatures, or overplanting.
A wide range of variation was observed among ten wheat (Triticum aestivum) varieties for eleven quantitative characters. The results of the data revealed that variance due to treatment was highly significant for all the characters. This suggested that there were inherent genetic differences among the genotype. During selection, the phenotypic variance was higher than the genotypic variance for all the characters; this indicated the influence of environmental factors on these traits. Similar findings were earlier reported by Subhani et al. (2011) and Shabbir et al. (2012). The estimates of the phenotypic coefficient of variation (PCV) were higher than the corresponding genotypic coefficient of variation (GCV) for all characters. Among all traits, grain yield and number of spikes showed a high value of the genotypic coefficient of variation (GCV) and phenotypic coefficient of variance (PCV). This indicated that the influence of the environment in the genotype is responsible for the variation in performance. The weight of ten spikes, number of grains per ten spikes, and tillers per m2 exhibited a high estimate of the f phenotypic coefficient of variation (PCV), the high value of the phenotypic coefficient of variation (PCV), and genotypic coefficient of variation (GCV) for this trait, it can be suggested that there would be the possibility of yield improvement through the selection of these traits. Plant height and 1000 grain weight exhibited moderate phenotypic coefficient of variation (PCV), while the spike length, 50% plant heading, and physiological maturity exhibited a low phenotypic coefficient of variation (PCV). The weight of ten spikes exhibited moderate genotypic coefficient of variation (GCV) while plant height, 50% heading, physiological maturity, number of tillers per m2, spike length, number of grains per ten spikes, 1000 grain weight exhibited low genotypic coefficient of variation (GCV).
It can be concluded from the morphological study as evidenced by the analysis of variance and other statistical tools employed that significant differences occurred among the genotypes for all the traits and selection based on yield and yield component traits would be effective. Consequent to the influence of the environment and other climatic factors on wheat production in this part of the country, seasons and timing for trials could be varied for enhanced productivity of the wheat genotypes. From the observed results in this study, ATTILA-7(CHECK), HUBARA, and SERI.IB showed some degree of adaptability to Akungba-Akoko. Hence, further trials need to be conducted to actually ascertain the adaptability profile of these genotypes.
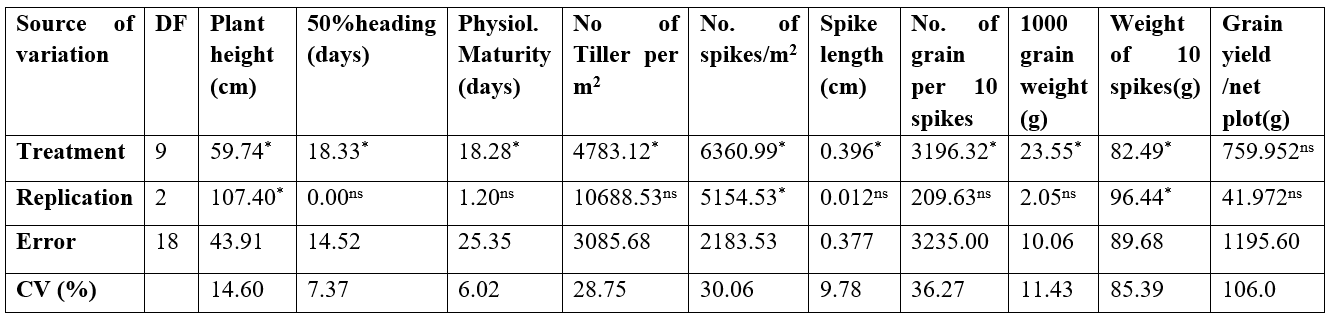
Ns: Not significant; *: significant at 5%; **: significant at 1%. CV: Coefficient of variation.
Table 2. Analysis of variance for quantitative traits of ten wheat genotypes
Means with the same alphabet within a column are not significantly different from one another
Table 3. Mean performance on ten quantitative traits of wheat genotypes
|
TRAIT |
GCV (%) |
PCV (%) |
H2B (%) |
GA (%) |
GAM (%) |
|
|
|
|
|
|
|
|
Plant height (cm) |
12.39 |
16.0 |
59.15 |
4.87 |
10.73 |
|
50%heading (days) |
7. 43 |
7.00 |
99.53 |
5.42 |
10.48 |
|
Physiol. Maturity (days) |
5.29 |
6.00 |
91.91 |
4.64 |
5.55 |
|
Tiller per m2 |
28.92 |
33.00 |
75.12 |
8.89 |
4.60 |
|
No. of spikes/m2 |
23.13 |
40.00 |
34.27 |
4.46 |
2.87 |
|
Spike length (cm) |
9.52 |
10.00 |
5.56 |
0.37 |
5.87 |
|
No. of grain per 10 spikes |
18.91 |
35.04 |
29.12 |
3.55 |
2.26 |
|
1000 grain weight (g) |
9.20 |
15.77 |
33.95 |
2.78 |
11.67 |
|
Weight (g) of 10 spikes |
81.99 |
84.30 |
94.18 |
17.81 |
160.60 |
|
Grain yield (g/net plot) |
92.31 |
95.65 |
93.15 |
18.77 |
57.36 |
GCV: Genotypic coefficient of variation; PCV: Phenotypic coefficient of variation; H2B: Broad-sense heritability; GA: Genetic advance; GAM: Genetic advance as percent of the mean
Table 4. Estimates of genetic parameters on ten wheat genotypes
*: significant at 5%; **: significant at 1
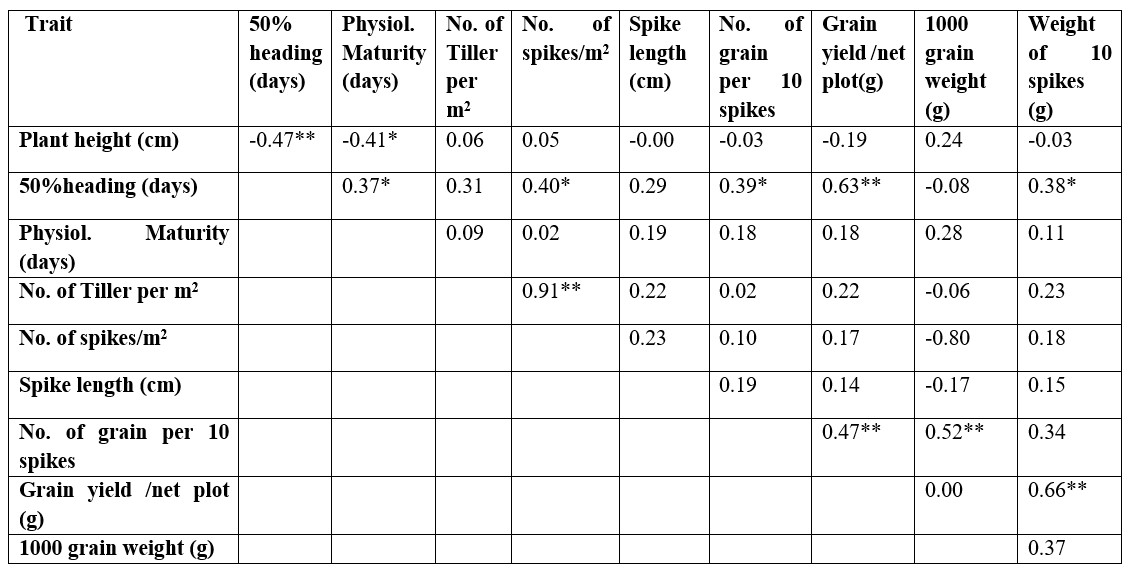
Table 5. Correlation coefficient analysis on quantitative traits in ten wheat genotype