Agricultural Research Pesticides and Biofertilizers
OPEN ACCESS | Volume 6 - Issue 1 - 2026
ISSN No: 2994-0109 | Journal DOI: 10.61148/2994-0109/ARPB
Ahmed A. El-Shewy, Mohamed A. Seif El-Yazal*, Abrahim F.M. Abd El-Gawwad, Mostafa M. Rady
Botany Department, Faculty of Agriculture, Fayoum University.
*Corresponding authors: Mohamed A. Seif El-Yazal, Botany Department, Faculty of Agriculture, Fayoum University.
Received: December 13, 2021
Accepted: December 30, 2021
Published: January 12, 2022
Citation: Ahmed A. El-Shewy, Mohamed A. Seif El-Yazal, Abrahim F.M. Abd El-Gawwad, Mostafa M. Ra. (2022) “Integrative application of soil P-solubilizing bacteria and foliar nano-P improves growth and yield and reduces oxidative stress in Phaseolus vulgaris plants grown under calcareous soil conditions.”, Journal of Agricultural Research Pesticides and Biofertilizers, 3(2); DOI:http;//doi.org/01.2022/1.1054
Copyright: © 2022. Mohamed A. Seif El-Yazal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Two pot experiments were conducted in fall season of 2018 and summer season of 2019 in a greenhouse, with climatic conditions of 20.2 ± 3.0 °C as average day/night temperatures and 65.7 ± 8.8% as average relative humidity, at the Experimental Farm of the Faculty of Agriculture, Fayoum, Egypt. Healthy, uniform seeds of Phaseolus vulgaris, cv. Bronko were planted in plastic pots filled in equal quantities (12 kg) with calcareous soil (22% CaCO3). Soil enzyme activities (e.g., phosphatase and phytase) were significantly increased by inoculating the tested soil with phosphate-solubilizing bacteria (PSB) in both seasons of study. Inoculation of the calcareous soil with PSB and/or foliar application of Phaseolus vulgaris plants with MAP or NP resulted in significant increases in growth characteristics (shoot length, No. of leaves plant‒1, leaves area plant‒1, shoot fresh and dry weights), pod and seed yield components (average pod weight, No. of pods plant−1, green pods weight plant‒1, and dry seeds weight plant‒1), photosynthetic efficiency (total chlorophylls content, Fv/Fm, and net photosynthesis rate), relative water content, and membrane stability index, while the contents of carotenoids, lipid peroxidation (malondialdehyde; MDA), oxidative stress biomarkers (O2•‒ and H2O2), and electrolyte leakage were decreased significantly compared to the control. PSB+NP was the best treatment in all growing seasons. Based on the study results, it can be concluded that inoculation of calcareous soil with PSB in integration with foliar spray with NP significantly improved growth and productivity of Phaseolus vulgaris plant grown under high carbonate (CaCO3; calcareous state) stress by inducing decreases in reactive oxygen species production that coincided with a decrease in lipid peroxidation and electrolyte leakage.
Introduction:
Globally, especially in developing countries, the demand for food is growing rapidly, where croplands and resources scarcely contribute to the effective production of the strategic crops, which are needed to meet this pressing demand for food. There is an urgent need to maximize agricultural productions in sustainable techniques in defected soils such as calcareous soils. Among these technologies, the use of effective agricultural bio-systems that take into account the biochemical diversity of entire agricultural ecosystems and their capacity to mitigate the adverse effects of low soil fertility and abiotic stresses, including high carbonate content in soils; calcareous state (Timmusk et al., 2017; Bargaz et al., 2018; Belal et al., 2019). In this regard, the issue of global food security will promote dependence on innovation, development, and delivery of technologies that elevate food production, while confirming sustainable intensification of agriculture. One of the adopted innovative and effective technologies is the integrated bio- (e.g., phosphate solubilizing bacteria; PSB) and chemical fertilization (e.g., phosphorus; P) strategy that provide highly valuable information for monitoring and securing crop productivity (Salih et al., 1989; Sundara et al., 2002; Shi et al., 2017).
High carbonate content (e.g., calcareous soils) is a factor that limits the availability of mineral nutrients, especially phosphorus (P) and agricultural productivity (Belal et al., 2019). Calcareous soil contains a large amount of calcium carbonate (CaCO3), which predominates in problems of agricultural land use (FAO, 2016). Leytem and Mikkelsen (2005) have defined calcareous soils as they contain large amounts of free excess lime (e.g., CaCO3 or MgCO3). They have also defined calcareous soils as soils containing more than 14–17% CaCO3 or more than 4–7% active CaCO3 with reference to the hydraulic properties of the entire soil. These soils are very widespread in Mediterranean regions and represent the dominant type of soil in many dry (e.g., arid and semi-arid) climates (Leytem and Mikkelsen, 2005). In addition, the occurrence of these soils have been verified in arid (arid and semi-arid) and humid (humid and sub-humid) areas (Brady and Weil, 2008). Calcareous soils are evaluated as having a few‒95% CaCO3 and covering more than 30% of the Earth’s surface (Marschner, 1995). High carbonates control the chemistry of these soils, which have alkaline reactions. In most calcareous soils, carbonates negatively affect the pH value to be around 7.5‒8.5, making nutrients unavailable to plants, adversely influence the physical properties (e.g., availability of soil water to plants and crust of soil surface), and detrimentally affect, directly or indirectly, the chemical properties (e.g., availability of macro- and micro-nutrients; N, P, K, Mg, Zn, Cu, and Fe) (Marschner, 1995). All these harmful effects of high carbonates lead to detrimental effects on soil structure and fertility associated with plant growth (FAO, 2016). In addition, soils with high CaCO3 and pH, and low organic matter, enzymatic activity and available nutrients. These undesirable properties make the soil defective and less productive. Therefore, to cultivate these soils, many challenges should be addressed. Among these challenges, low CEC, low water-holding capacity, low organic matter (OM) and clay contents, poor structure, low available nutrients, especially P and micronutrients, nutritional imbalances, nutrient loss by leaching or deep percolation, N fertilizer loss, surface crusting and cracking, serious compaction, high pH, and high infiltration rate (El-Hady and Abo-sedera, 2006). However, in the presence of phosphate solubilizing micro-organisms such as phosphate solubilizing bacteria (PSB) and the availability of P, high carbonates content conditions (calcareous state) tend to repair.
PSB play a pivotal role in solubilizing soil P and increasing its bioavailability for plants through transforming insoluble P to available P in the soil, improving fertilizer use efficiency and crop productivity (Hu et al., 2012; Shi et al., 2017). Application of PSB in combination with chemical P fertilizer into defected soil (e.g., calcareous) is an integrated biotechnology practice for comprehensive management and improvement of soil fertility (Sundara et al., 2002; Shi et al., 2017). Previous investigations concerning the application of PSB to disordered soils have focused mostly on increasing availability of soil P and biological activity with reference to crop yield. In addition, the integrated application of bio-fertilizer PSB+chemical fertilizer+organic fertilizer was more useful for defected soil repair (Liang et al., 2010; Shi et al., 2017).
To increase its use efficiency (PUE), P can be used in nanoparticles form (the so-called "smart fertilizer"), especially as foliar application. Nano-fertilizers are defined as materials with a single-unit ranging in size from 1 to 100 nm in at least one dimension. These nanoparticles have a positive and negative charge on the same particle that improves the uptake of other nutrients by retaining those nutrients in the soil against various losses (Liu and Lal, 2014).
Food legumes are an important constituent in promoting sustainable agriculture and human nutrition worldwide. Legumes are a rich source of protein, especially common bean (Phaseolus vulgaris L.), which represents 50% of the total grain legumes consumed globally (Broughton et al., 2003). The cultivation of legumes is beneficial to non-legume crops through numerous agro-ecological contributions such as biological fixation of N, enhancement of soil fertility and production of N-rich green manure (Isaac et al., 2011). However, the nutritional, ecological and economic contributions of legumes are often compromised by their sensitivity to environmental stresses that reduce crop growth and productivity (Scheelbeek et al., 2018). Among these environmental stresses, the damaging biotic and abiotic constraints of the calcareous soil such as limited availability of water, scarcity of nutrients (especially P), increased compaction of soil, increase of carbonates, and decreased fertility and defected structure of soil (Belal et al., 2019).
Therefore, this study was planned to examine the effect of inoculation of calcareous soil (22% CaCO3) with PSB biofertilizer and foliar treatment of Phaseolus vulgaris plants with some P forms (e.g., mono-ammonium phosphate; MAP and P in nanoparticles) on plant performance (growth and yields), physiological attributes, and accumulation of oxidative stress biomarkers. In addition, Phaseolus vulgaris crop was selected for this study because it is one of the most sensitive crops to different types of environmental stressors (Sultana et al., 2014; Bargaz et al., 2016).
Materials and Methods:
Growing conditions of plant material, treatments, and experimental layout:
Two pot experiments were conducted in two different growing seasons; fall, 2018 and summer, 2019 using an open greenhouse at the experimental farm of the Faculty of Agriculture, Fayoum (29°17ʹ06” N 30°54ʹ55” E), Egypt. The climatic conditions were 12.3 to 28.1°C as daily temperatures with an average of 20.2 ± 3.0°C, and 52.4 to 79.0% as daily relative humidity with an average of 65.7 ± 8.8%.
Healthy and uniform seeds of common bean (Phaseolus vulgaris) cv. Bronko were purchased from the Horticulture Research Institute, Agricultural Research Center, Ministry of Agriculture, Giza, Egypt. The seeds were surface sterilized with 1% (v/v) NaOCl for 5 min and then thoroughly washed several times with double-distilled water. The seeds were left to air dry for 1 h and then prepared for sowing. Plastic pots of 35 cm in inner diameter and 32 cm depth were filled in equal quantities (12 kg) with soil that characterized as calcareous (21.8 ‒ 22.2% with an average of 22% CaCO3 for all growing seasons). Based on the physicochemical analyses (Page et al., 1982; Klute and Dirksen, 1986) of this calcareous soil for all preliminary and main studies, it was clay in texture. The physicochemical analyses of this tested soil are shown in Table 1.
Table 1: Physical and chemical properties of the experimental soil used for two different seasons before beginning the experiments
|
Parameter |
Fall season of 2018 |
Summer season of 2019 |
|
Clay |
49.8 |
50.2 |
|
Silt |
30.2 |
30.5 |
|
Sand |
20.0 |
19.3 |
|
Soil texture |
Clay |
|
|
pH |
8.18 |
8.11 |
|
EC (dS m−1) |
2.28 |
2.19 |
|
Organic matter (%) |
0.74 |
0.71 |
|
CaCO3 (%) |
21.8 |
22.2 |
|
CEC (cmolc kg−1) |
5.79 |
5.66 |
|
Available macro- and micronutrients (mg kg−1 soil) |
||
|
Available N |
12.4 |
12.8 |
|
Available P |
5.41 |
5.60 |
|
Available K |
24.5 |
26.4 |
|
Available Fe |
5.91 |
6.22 |
|
Available Mn |
5.04 |
5.12 |
|
Available Zn |
3.50 |
3.34 |
"dS m−1" means decisiemens per meter, "CEC" means cation exchange capacity, "cmolc kg−1" means centimole of cation exchange capacity per kilogram soil, and "mg kg−1" means milligram per kilogram.
A total number of 120 pots were used for six treatments for each growing season. Each treatment needed to 20 pots as four replicates, 5 pots for each. The calcareous soil of 60 pots (3 treatments) was inoculated by phosphate solubilizing bacteria (PSB; a mixture of Pseudomonas mallei and Pseudomonas cepaceae) and the soil of the other 60 pots (3 treatments) was not inoculated, forming 6 treatments as follows: (1) control (without any treatments), (2) soil inoculated with PSB, (3) soil without inoculation + spraying plants with 1.0 g L‒1 MAP, (4) soil without inoculation + spraying plants with 0.1 g L‒1 NP, (5) soil inoculated with PSB + spraying plants with 0.5 g L‒1 MAP, and (6) soil inoculated with PSB + spraying plants with 0.05 g L‒1 NP. The MAP fertilizer (Great Neck, NY 11021, USA) used contains N, P, and K at a ratio of 12, 61, and 0 %, respectively. It is 100% water soluble with low pH. The amount of N found in MAP was calculated and added (as foliar spray) to plants in all treatments that did not receive MAP to offset the effect of N in all treatments. NP was prepared in the laboratory using ball-milling (Photon Company, Egypt) following Eleyan et al. (2018). Transmission Electron Microscopy (TEM) was used to investigate and measure NP particle size (4.92‒8.62 nm) using JEOL transmission electron microscope (JEM-1400 TEM, Japan) following Wang et al. (2014). The soil in all pots received the full recommended dose of NPK and organic manures. Each pot (12 kg soil) received 3.6 g of ammonium sulfate (20% N) + 2.4 g of calcium superphosphate (15% P2O5) + 1.2 g of potassium sulfate (48% K2O).
In both experimental seasons, experiments was repeated 3 times in a layout itemized depending on the completely randomized design (CRD) with 20 pots for each treatment. Pots of all treatments were rotated (from place to place) every 2 days to ensure fairness in the distribution of light and sunlight intensity for all plants. In each pot, 10 homogenous seeds were sown and after full emergence, thinning was attained to maintain 3 uniform seedlings per pot. All pots were irrigated day by day. The types of phosphorus (MAP and NP) were sprayed for plants two times at 25 and 40 days after sowing (DAS). A handheld manual sprayer (model 0417.02.00; Guarany Ind. & Com. Ltd) was used to spray the different solutions of MAP and NP on the upper leaf surface until run-off (approximately 120 ml per pot), and few drops of Tween-20 were added to the spray solutions as a surfactant. In addition, all agricultural practices were applied as recommended for commercial common bean production.
At 50 DAS, common bean plants (n = 9) were harvested to assess growth, parameters of plant physiological attributes, and oxidative stress biomarkers. At the end of experiments, the marketable green pods yield per plant was assessed and after drying the pods, seed yield per plant was estimated.
Isolation, identification, and application of phosphate-solubilizing bacteria (PSB) inoculants:
The PSB (a mixture of Pseudomonas mallei and Pseudomonas cepaceae) were produced using the Nutrient Broth (NB) medium. This PSB inoculant was isolated from wheat rhizosphere in the Microbiology Laboratory, Faculty of Agriculture, Fayoum University. The isolates were molecularly identified in a specialized laboratory, National Research Center, Cairo, Egypt. The oligonucleotide primers used for specific PCR were as follows:
|
Target species |
Primer |
23S rDNA helices containing target position |
Sequence |
Size of PCR product (bp) |
Annealing temp (°C) |
|
P. mallei |
M 23-2 |
78ab |
5'-CAC CGA AAC TAG CA-3' |
526 |
47 |
|
P. Cepaceae |
CVP 23-2 |
78ab |
5'-CAC CGA AAC TAG CG-3' |
526 |
47 |
The bacteria (P. mallei and P. cepaceae) were tested for its ability to solubilize P and to reduce pH in culture conditions and microcosms, and also identified and reported as PSB and plant growth-promoting rhizobacteria (PGPR). The two isolates exhibited no antagonistic activity against each other. Subsequently, the obtained PSB inoculant was added to a carrier material, which was a mixture of compost and peat at a ratio of 1: 1. This carrier material was encapsulated using aluminum foil and sterilized using an autoclave. Thereafter, the PSB inoculant was added at a ratio of 10% to the carrier material (e.g., 1 L of inoculant for each 10 kg of carrier material). The PSB inoculant was packed and maintained until use. At 48 h prior to sowing, the treatment with the PSB inoculant was applied to the calcareous soil at 1 g (0.1 mL of net PSB) kg‒1 soil.
Assaying of soil enzymatic activities:
After harvest of Phaseolus vulgaris, soil samples were collected from pots in which soil was inoculated with PSB in addition to soil samples taken prior to inoculation with PSB. Replicates of each soil sample were well mixed and passed through a < 2-mm sieve to discard pebbles and plant stubbles. Soil samples were stored at 4 °C in a refrigerator until use to determine soil enzymatic activities. Soil phosphatase activity was assayed colorimetrically using disodium phenyl phosphate (Guan, 1986). Assaying phytase activity in soil solutions and suspensions was performed using a sample: buffer ratio of 1:1. Assays were conducted against an InsP6 substrate for 60 min at 37 °C at 2 mM as a final concentration, pH 5.5, with 15 mM of 2-morpholinoethanesulfonic acid (MES). Prior to use, the stock solution (InsP6; 20 mM) was acidified to pH 5.5 with 10 M HCl, and the filtrate was sterilized (0.22 mm) (George et al., 2005; Giaveno et al., 2010). The reactions were stopped with an equal volume of 10% TCA (trichloroacetic acid). Samples were then centrifuged at 3,800 × g for 5 min. Thereafter, P concentration was determined in the supernatant using malachite green (Irving and McLaughlin, 1990).
As P released during 60 min assay, phytase activity (nKat g–1 soil) was calculated as follows:
Phytase activity (nKat g–1 soil) = (P × D × V × 16.67) ÷ (T × 31),
where P is the P concentration (mg L–1), D is the divide ratio, V is the volume (mL), and T is the incubation time (60 min).
Determination of growth and yield parameters:
At 50 DAS, leaves on each plant were counted, shoot length was assessed by using a meter scale, and leaves area of each plant was evaluated by using a graph sheet. Fresh weight of plant shoot (n = 9) was assessed using an electric balance. Shoots were then oven-dried at 70 °C for 24 h and then weighed for dry weights.
At merchantable green pod stage (62 ‒ 70 DAS), pods were picked from random 25 plants to measure number of pods per plant, average pod weight, and weight (g) of green pods per plant by using an electric balance. At 80 DAS, pods were picked from the remaining (n = 25) plants and left to air-dry for 72 h. Seeds were then extracted from dry pods to assess the average weight (g) of dry seeds per plant.
Determination of leaf photosynthetic pigments, photosynthetic (PSII) efficiency (Fv/Fm), and net photosynthesis rate (Pn):
Photosynthetic pigments (total chlorophylls and carotenoids) contents were determined using the acetone extract method (Arnon, 1949). Absorbance readings were recorded at 480 nm, 645 nm, 663 nm using a spectrophotometer (Beckman 640D, USA) to calculate the contents of pigments in mg g‒1 leaf fresh weight.
Efficiency of photosynthesis (PSII) was measured on two different sunny days using a portable fluorometer (Handy PEA, Hansatech Instruments Ltd, Kings Lynn, UK). One leaf (the same age) was chosen per plant from five plants from each treatment. A total of 10 measurements per treatment were made, including maximum quantum yield of PS II Fv/ Fm was calculated as; Fv/ Fm = (Fm - Fo)/Fm (Maxwell and Johnson, 2000).
By using PAM chlorophyll fluorimeter (H. Walz, Effeltrich, Germany), net photosynthesis rate (Pn) was assessed in upper fully expanded leaf tissue (Li et al., 2007). The infrared gas analyzer (LCA-4 model, Anal. Dev. Co., Hoddesdon, England) was used to measure the Pn, which was performed in upper fully expanded leaf tissue.
Assessment of leaf relative water content (RWC), membrane stability index (MSI), and electrolyte leakage (EL):
After excluding leaf midrib, 2 cm-diameter discs were taken for RWC determination (Osman and Rady, 2014). Discs were weighed for fresh mass (FM) and immersed, immediately, in deionized water in dark for 24 h. Water-saturated discs were blotted dry from adhering water drops for recording the turgid mass (TM). Discs were then dried at 70 °C for 48 h for dry mass (DM) assessment. The percentage of RWC was calculated using the following equation:
RWC (%) = (FM – DM)/ (TM – DM) × 100
After excluding leaf midrib, a duplicate 0.2 g leaf sample was taken in test tubes with 10 ml of deionized water to determine leaf MSI (Rady, 2011). At 40 °C, a sample was heated for 30 min with a water bath. Solution electrical conductivity (C1) was taken. At 100 °C, the other sample was boiled for 10 min. Solution conductivity (C2) was also measured. The percentage of MSI was calculated using the following equation:
MSI (%) = 1 – (C1 / C2) × 100
Total inorganic ions that leaked from leaves termed as EL were assessed with the (Rady, 2011) procedure. Twenty discs were immersed in 10 ml deionized water in a boiling tube and solution electrical conductivity (C1) was measured. Tube content was then heated to 45 °C – 55 °C for 30 min using a water bath. Solution electrical conductivity (C2) was scored. At 100 °C, tube content was boiled for 10 min and solution electrical conductivity (C3) was also recorded. The percentage of EL was calculated using the following equation:
EL (%) = [(EC2 − EC1) / EC3] × 100
Determination of lipid peroxidation (assessed as malondialdehyde; MDA) and oxidative stress biomarkers (hydrogen peroxide; H2O2, and superoxide; O2•‒) contents:
The methods described in Velikova et al. (2000), Kubis (2008), and Madhava Rao and Sresty (2000) were utilized to determine the contents of H2O2, O2•‒, and lipid peroxidation (in terms of malondialdehyde; MDA), respectively. For H2O2 content, the absorbance of samples was read spectrophotometrically at 390 nm and the content (µmol g‒1 FW) was calculated using a suitable standard curve. For O2•− content (µmol g−1 FW), fragments (1 × 1 mm) of samples (100 mg) were flooded in a mixture of buffer (K-phosphate, 10 mM, pH 7.8), NBT (0.05%) and NaN3 (10 mM) for 1 h at room temperature. After heating at 85 °C for ¼ h and then cooling rapidly, optical density was read colorimetrically at 580 nm. For lipid peroxidation, it was evaluated as a content of malondialdehyde (MDA) that formed due to stress. The extinction coefficient 155 mM‒1 cm‒1 was applied to calculate MDA content.
Statistical analysis:
Data are presented in terms of means (± SE; standard error). The completely randomized design (CRD) was the layout of the preliminary and main studies. ANOVA was followed to statistically analyses of all data. Tukey’s HSD test (SPSS 14.0; SPSS Chicago, IL, USA) was then applied and P ≤ 0.05 was used to analyze the significant differences among treatments.
Results:
Soil enzymatic activities:
Soil enzyme activities (e.g., phosphatase and phytase) have been increased by inoculating the tested calcareous soil with phosphate-solubilizing bacteria (PSB) both in the fall season of 2018 and summer season of 2019 (Table 2). The increases were 153 and 158% for phosphatase activity, and 143 and 134% for phytase activity in both seasons, respectively.
Table 2: Physical and chemical properties of the experimental soil used for two different seasons before and after its inoculation with phosphorus-solubilizing bacteria (PSB)
|
Parameter |
Prior to soil inoculation with PSB |
After soil inoculation with PSB |
||
|
Fall season of 2018 |
Summer season of 2019 |
Fall season of 2018 |
Summer season of 2019 |
|
|
Clay |
49.8 |
50.2 |
49.9 |
50.4 |
|
Silt |
30.2 |
30.5 |
30.5 |
30.6 |
|
Sand |
20.0 |
19.3 |
19.6 |
19.0 |
|
Soil texture |
Clay |
|||
|
pH |
8.18 |
8.11 |
7.91 |
7.86 |
|
EC (dS m−1) |
2.28 |
2.19 |
2.31 |
2.24 |
|
Organic matter (%) |
0.74 |
0.71 |
0.80 |
0.82 |
|
CaCO3 (%) |
21.8 |
22.2 |
19.7 |
19.9 |
|
CEC (cmolc kg−1) |
5.79 |
5.66 |
6.82 |
6.80 |
|
Available macro- and micronutrients (mg kg−1 soil) |
||||
|
Available N |
12.4 |
12.8 |
14.2 |
14.6 |
|
Available P |
5.41 |
5.60 |
9.74 |
9.86 |
|
Available K |
24.5 |
26.4 |
27.2 |
28.9 |
|
Available Fe |
5.91 |
6.22 |
6.21 |
6.31 |
|
Available Mn |
5.04 |
5.12 |
5.18 |
5.23 |
|
Available Zn |
3.50 |
3.34 |
3.62 |
3.56 |
|
Soil enzymatic activities |
||||
|
Phosphatase (mg P2O5 100 g−1 h−1) |
0.53 |
0.55 |
1.34 |
1.42 |
|
Phytase (nKat g–1 soil) |
5.71 |
6.03 |
13.9 |
14.1 |
Meaning of abbreviations: "dS m−1" means decisiemens per meter, "CEC" means cation exchange capacity, "cmolc kg−1" means centimole of cation exchange capacity per kilogram soil, "mg kg−1" means milligram per kilogram, and "mg P2O5 100 g−1 h−1" means milligram of phosphorus pentoxide per 100 gram soil per hour.
Growth characteristics:
Inoculation of calcareous soil with PSB and/or foliar application of Phaseolus vulgaris plants with MAP or NP resulted in significant increases in growth characteristics [shoot length (SL), No. of leaves plant‒1 (NLP), leaves area plant‒1 (LAP), shoot fresh weight (SFW), and shoot dry weight (SDW)] compared to control (Table 3). Integrative treatments (PSB+MAP and PSB+NP) were more effective than individual treatments (PSB, MAP, or NP) in improving the above growth attributes. In addition, PSB+NP was better than PSB+MAP, improving SL, NLP, LAP, SFW, and SDW by 69 and 67%, 66 and 51%, 84 and 85%, 65 and 64%, and 81 and 83% in the fall season of 2018 and summer season of 2019, respectively.
Table 3: Effect of soil application with phosphorus-solubilizing bacteria and foliar application with traditional (MAP; mono-ammonium phosphate) or nano phosphorus (NP) on growth traits of common bean plants (cv. Bronco) grown under calcareous soil conditions
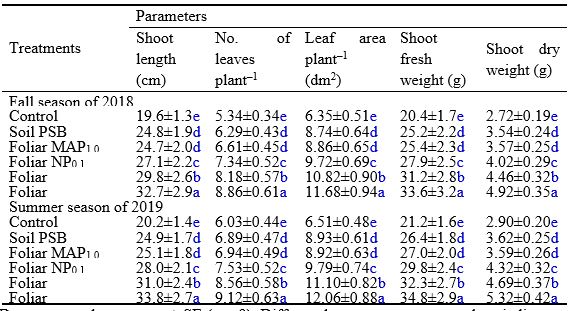
Data presented are means ± SE (n = 9). Different letters next to mean values indicate significant differences at P ≤ 0.05. All pots of all treatments, including the control, received full recommended doses of NPK fertilizers for common bean production on calcareous soils.
Pod and seed yields and their components:
Inoculation of calcareous soil with PSB and/or foliar application of Phaseolus vulgaris plants with MAP or NP resulted in significant increases in pod and seed yields and their components [average pod weight (APW), No. of pods plant−1 (NPP), green pods weight plant‒1 (PWP), and dry seeds weight plant‒1 (SWP)] compared to control (Table 4). Integrative treatments (PSB+MAP and PSB+NP) were more effective than individual treatments (PSB, MAP, or NP) in improving the above yields components. Additionally, PSB+NP was better than PSB+MAP, increasing APW, NPP, PWP, and SWP by 119 and 108%, 159 and 157%, 469 and 433%, and 447 and 472% in the fall season of 2018 and summer season of 2019, respectively.
Table 4. Effect of soil application with phosphorus-solubilizing bacteria and foliar application with traditional (MAP; mono-ammonium phosphate) or nano phosphorus (NP) on yield components of common bean plants (cv. Bronco) grown under calcareous soil conditions
|
Treatments |
Parameters |
|||
|
Average pod weight (g) |
No. of pods plant−1 |
Pods weight plant−1 (g) |
Seeds weight plant−1 |
|
|
Fall season of 2018 |
||||
|
Control |
1.42±0.11f |
10.1±0.7e |
14.3±1.1f |
3.42±0.22e |
|
Soil PSB |
2.08±0.15e |
15.2±1.1d |
31.6±2.4e |
6.92±0.39d |
|
Foliar MAP1.0 |
2.29±0.17d |
15.4±1.0d |
34.5±2.8d |
7.59±0.58d |
|
Foliar NP0.1 |
2.58±0.19c |
18.6±1.3c |
48.0±3.4c |
10.56±0.64c |
|
Foliar MAP0.5+Soil PSB |
2.84±0.20b |
23.5±1.9b |
66.7±5.7b |
14.67±0.89b |
|
Foliar NP0.05+Soil PSB |
3.11±0.25a |
26.2±2.1a |
81.4±6.5a |
18.72±1.28a |
|
Summer season of 2019 |
||||
|
Control |
1.55±0.10e |
10.5±0.9e |
16.3±1.2e |
3.51±0.22e |
|
Soil PSB |
2.15±0.14d |
16.2±1.3d |
34.8±2.8d |
7.66±0.45d |
|
Foliar MAP1.0 |
2.21±0.15d |
15.9±1.3d |
35.1±2.9d |
7.72±0.48d |
|
Foliar NP0.1 |
2.61±0.18c |
19.2±1.7c |
50.1±3.2c |
11.02±0.63c |
|
Foliar MAP0.5+Soil PSB |
2.90±0.20b |
24.1±2.1b |
69.9±4.4b |
15.42±1.15b |
|
Foliar NP0.05+Soil PSB |
3.22±0.24a |
27.0±2.4a |
86.9±6.1a |
20.06±1.42a |
Data presented are means ± SE (n = 9). Different letters next to mean values indicate significant differences at P ≤ 0.05. All pots of all treatments, including the control, received full recommended doses of NPK fertilizers for common bean production on calcareous soils.
Leaf photosynthetic pigments and photosynthetic efficiency:
Inoculation of calcareous soil with PSB and/or foliar application of Phaseolus vulgaris plants with MAP or NP resulted in significant increases in photosynthetic efficiency (total chlorophylls content, Fv/Fm, and net photosynthesis rate; Pn), except for carotenoids content, which was decreased significantly compared to control (Table 5). Integrative treatments (PSB+MAP and PSB+NP) were more effective than individual treatments (PSB, MAP, or NP) in improving the above attributes. In addition, PSB+NP was better than PSB+MAP, increasing total chlorophylls content, Fv/Fm, and Pn by 139 and 143%, 76 and 76%, and 97 and 98% in both fall (2018) and summer (2019) seasons, respectively.
Table 5: Effect of soil application with phosphorus-solubilizing bacteria and foliar application with traditional (MAP; mono-ammonium phosphate) or nano phosphorus (NP) on leaf photosynthetic pigments and chlorophyll fluorescence parameters of common bean plants (cv. Bronco) grown under calcareous soil conditions
|
Treatments |
Parameters |
|||
|
Total Chlorophylls (mg−1 FW) |
Total carotenoids (mg−1 FW) |
Efficiency of PSII (Fv/Fm) |
Net photosynthesis rate (Pn; mmol m−2 s−1) |
|
|
Fall season of 2018 |
||||
|
Control |
0.76±0.02f |
0.46±0.01a |
0.50±0.01e |
8.04±0.18e |
|
Soil PSB |
1.02±0.03e |
0.41±0.01b |
0.61±0.02d |
10.52±0.21d |
|
Foliar MAP1.0 |
1.24±0.04d |
0.38±0.01c |
0.64±0.02d |
10.80±0.23d |
|
Foliar NP0.1 |
1.34±0.04c |
0.38±0.01c |
0.71±0.02c |
12.18±0.25c |
|
Foliar MAP0.5+Soil PSB |
1.60±0.05b |
0.35±0.01d |
0.81±0.03b |
14.12±0.29b |
|
Foliar NP0.05+Soil PSB |
1.82±0.05a |
0.32±0.00e |
0.88±0.03a |
15.82±0.31a |
|
Summer season of 2019 |
||||
|
Control |
0.80±0.03e |
0.51±0.02a |
0.51±0.01f |
8.15±0.15e |
|
Soil PSB |
1.12±0.03d |
0.44±0.01b |
0.60±0.02e |
11.08±0.20d |
|
Foliar MAP1.0 |
1.32±0.04c |
0.43±0.01b |
0.66±0.02d |
11.32±0.21d |
|
Foliar NP0.1 |
1.36±0.04c |
0.38±0.01c |
0.73±0.02c |
12.84±0.25c |
|
Foliar MAP0.5+Soil PSB |
1.66±0.05b |
0.33±0.01d |
0.83±0.03b |
14.56±0.30b |
|
Foliar NP0.05+Soil PSB |
1.94±0.05a |
0.30±0.00e |
0.90±0.03a |
16.10±0.33a |
Data presented are means ± SE (n = 9). Different letters next to mean values indicate significant differences at P ≤ 0.05. All pots of all treatments, including the control, received full recommended doses of NPK fertilizers for common bean production on calcareous soils.
Relative water content, membrane stability index and electrolyte leakage:
Soil inoculation by PSB and/or plant treatment by MAP or NP conferred significant increases in relative water content (RWC) and membrane stability index (MSI), and awarded significant decreases in electrolyte leakage (EL) in Phaseolus vulgaris plants compared to control (Table 6). PSB+MAP or PSB+NP was more effective than individual treatments in improving the above attributes. Further, PSB+NP was better than PSB+MAP, increasing RWC and MSI by 72 and 67%, and 63 and 59%, and decreasing EL by 69 and 69% in both seasons, respectively.
Table 6. Effect of soil application with phosphorus-solubilizing bacteria and foliar application with traditional (MAP; mono-ammonium phosphate) or nano phosphorus (NP) on leaf relative water content (RWC) and oxidative stress (membrane stability index; MDI, electrolyte leakage; EL, malondialdehyde content; MDA, superoxide content; O2•‒, and hydrogen peroxide content; H2O2) in common bean plants (cv. Bronco) grown under calcareous soil conditions
|
Treatments |
Parameters |
|||||
|
RWC (%) |
MSI (%) |
EL (%) |
MDA (mM g−1 FW) |
O2•‒ (mM g−1 FW) |
H2O2 (mM g−1 FW) |
|
|
Fall season of 2018 |
||||||
|
Control |
54.2±3.2e |
42.8±2.8e |
39.0±2.1a |
3.77±0.08a |
0.52±0.02a |
4.03±0.07a |
|
Soil PSB |
72.3±4.8d |
55.4±3.0d |
25.1±1.8b |
2.80±0.06b |
0.38±0.01b |
2.97±0.05b |
|
Foliar MAP1.0 |
73.0±5.0d |
56.1±3.3d |
24.9±1.6b |
2.76±0.06b |
0.37±0.01b |
2.94±0.05b |
|
Foliar NP0.1 |
79.2±5.4c |
60.1±3.7c |
19.7±1.4c |
2.28±0.04c |
0.29±0.01c |
2.41±0.04c |
|
Foliar MAP0.5+Soil PSB |
88.4±6.9b |
65.8±4.1b |
16.2±1.2d |
1.89±0.04d |
0.19±0.00d |
1.89±0.03d |
|
Foliar NP0.05+Soil PSB |
93.1±7.2a |
69.6±4.5a |
12.2±1.0e |
1.08±0.02e |
0.12±0.00e |
1.46±0.03e |
|
Summer season of 2019 |
||||||
|
Control |
56.3±4.1e |
44.4±2.2e |
38.4±2.2a |
3.54±0.07a |
0.49±0.01a |
3.92±0.08a |
|
Soil PSB |
71.9±5.3d |
56.3±2.8d |
28.1±2.0b |
2.60±0.05b |
0.33±0.01b |
2.90±0.06b |
|
Foliar MAP1.0 |
71.2±5.4d |
56.6±3.2d |
27.6±1.8b |
2.59±0.05b |
0.34±0.01b |
2.86±0.06b |
|
Foliar NP0.1 |
78.8±5.9c |
61.0±3.5c |
21.2±1.5c |
2.19±0.04c |
0.27±0.01c |
2.31±0.05c |
|
Foliar MAP0.5+Soil PSB |
86.3±6.8b |
66.1±4.3b |
16.0±1.2d |
1.73±0.03d |
0.15±0.00d |
1.68±0.03d |
|
Foliar NP0.05+Soil PSB |
94.0±7.4a |
70.6±4.8a |
11.8±0.9e |
1.02±0.02e |
0.10±0.00e |
1.39±0.03e |
Data presented are means ± SE (n = 9). Different letters next to mean values indicate significant differences at P ≤ 0.05. All pots of all treatments, including the control, received full recommended doses of NPK fertilizers for common bean production on calcareous soils.
Oxidative stress biomarkers and lipid peroxidation:
Soil inoculation by PSB and/or plant treatment by MAP or NP conferred significant decreases in lipid peroxidation (that measured as malondialdehyde; MDA content) and oxidative stress biomarkers (superoxide; O2•‒ and hydrogen peroxide; H2O2) in Phaseolus vulgaris plants compared to control (Table 6). PSB+MAP or PSB+NP was more effective than individual treatments in improving the above attributes. Moreover, PSB+NP was better than PSB+MAP, decreasing MDA, O2•‒, and H2O2 by 71 and 71%, 80 and 80%, and 64 and 65% in both seasons, respectively.
Discussion:
The calcareous soil used in this study has poor structure and undesirable properties such as high pH and calcium carbonate (CaCO3) content. It also contains a low content of organic matter (OM), available nutrients, especially P, and low enzymatic activities (Table 1). These undesirable properties indicate a low fertility with nutritional imbalance that makes the soil defective and less productive (Rady et al., 2020). These results are consistent with those obtained by El-Hady and Abo-Sedera (2006), Aboukila et al. (2018), and Rady et al. (2020). Under these harsh conditions, it is difficult to obtain a satisfactory level of yield, especially for Phaseolus vulgaris, a crop sensitive to various types of environmental stressors (Sultana et al., 2014; Bargaz et al., 2016), including high carbonate content (e.g., calcareous). Therefore, effective tools should be used to repair such harsh conditions of the tested calcareous soil.
Among a number of bacterial genera, Pseudomonas sp. are able to solubilize the metallic P-complex to release bioavailable P in orthophosphate form through specific mechanisms. These mechanisms mainly include organic acids and the production of siderophore and enzymes (e.g., phosphatase and phytase) that play a key role in hydrolyzing organic P forms (Table 1) into an absorbable form by the roots of plants (Rady et al., 2020).
Inoculation of a calcareous soil by phosphate-solubilizing bacteria (PSB) helped release of P from the fixation state to be available to plant roots (Rady et al., 2020). In addition, PSB effectively decreased CaCO3 and pH and increased OM, CEC, available nutrient, and enzymatic activity (e.g., phosphatase, and phytase) in the tested soil (Table 1). These improved properties by PSB make this soil productive, especially when PSB applied in integration with foliar application with P (mono-ammonium phosphate; MAP or nano-phosphorus; NP) for Phaseolus vulgaris plants (Tables 2–6).
In this study, PSB (a mixture of Pseudomonas mallei and Pseudomonas cepaceae) facilitated the transformation of insoluble P to soluble/available P in the tested soil (Table 2). This mechanism elevated soil P availability to roots of Phaseolus vulgaris plants, contributing to the increase in P content (Rady et al., 2020), growth, and productivity of plants (shoot length, number of leaves plant‒1, leaves area plant‒1, shoot fresh weight, shoot dry weight, average pod weight, pods number plant‒1, pods weight plant‒1, and seeds weight plant‒1; Tables 3 and 4). These results are consistent with those obtained by Hu et al. (2012), Shi et al. (2017), and Rady et al. (2020). This improved effect was more effective with the integrative treatment; PSB + foliar P application, especially NP, than individual treatment; PSB or foliar P (Tables 3 and 4). This superior effect of the integrative PSB+NP treatment is due to the efficacious capacity of PSB strains to solubilize P through the increase in the soil enzymes (e.g., phosphatase and phytase) as an effective mechanism, which increased the inorganic form of soil P to be available to plant roots (Tables 1 and 2) (Rady et al., 2020). In addition, NP could be an effective source of P nutrient as a soluble P fertilizer and plants can effectively take up P in nanoparticle formulation applied as foliar spray. It has been proved that P is important for the development and growth of plant cells, roots, flowers, fruits, and seeds. It also improves plant quality and strengthens plants against easily fall and diseases (Elfiati, 2005). In addition, P plays a pivotal role as a key ingredient in DNA, RNA, ATP, and phospholipids for healthy cell membranes (Schachtman et al., 1998; Rodríguez and Fraga, 1999; El-Ganaini et al.,2005).
Availability of soil P by PSB is one of the most important determinants of soil fertility in terms of increased contents of available nutrients and OM, and reduced content of CaCO3 (Table 2), which contributes to increased plant growth and productivity (Tables 3 and 4). Soil inoculation using PSB in integration with foliar spraying of P, especially NP, supports each other in supplying plants with nutrients, especially P for their life. Pseudomonas sp. work synergistically to produce phosphatases (Table 2) through mineralization and immobilization processes to transform organic P into inorganic form, so that the growth of Pseudomonas sp. can still be optimal from vegetative to harvest stage of plants (Fitriatin et al., 2014). As an efficient mechanism, the PSB strains secrete, quantitatively and qualitatively, organic acids (mainly as a gene-dependent; Zhen et al., 2016) in the soil to compete with P ions for the P adsorption sites, increasing P release in the soil for plants. PSB can promote the productivity of calcareous soil and elevate its biological activity (biochemical capacity of soil microorganisms and relevant enzyme; phosphatase and phytase activities) and available P content and other nutrients in such soil (Table 2). PSB enhance P use efficiency directly through exudation of organic acids and P-hydrolyzing phosphatase enzymes to improve P pool bioavailability, or indirectly through production of phytohormones, antifungal and toxin-resistance compounds, and other high value bioactive molecules which can help build a vigorous shoot/root system, especially under abiotic and biotic constraints (Shi et al., 2017) such as the problem under study; calcareous state. The influence of organic acids in solubilizing P is often attributed to reduced pH (from 8.11–8.18 to 7.86‒7.91) and cation chelating properties (Table 2), which were obtained due to PSB inoculation of the tested soil. Acidification of microbial cells perimeter results in the release of P anion by replacing H+ and Ca2+ (Behera et al., 2017) as a potential mechanism. Other potential mechanisms for solubilization of P in calcareous soil, the release of protons after NH4 assimilation by microbial cells, the production of inorganic acids (i.e., H2SO4 and HNO3), and the production of specific enzymes (Table 2) acting on amphiphilic fatty substances (Alori et al., 2017). In addition to microbial solubilization of mineral P, mineralization of organic P through microorganisms action also plays a pivotal role in P cycling, giving that organic P content in soil (often in inositol polyphosphates form) can account for between 30 and 50% of total P. Mineralization process of P is extensively controlled by specialized P-hydrolyzing enzymes produced by PSB such as phosphatases and phytases, which are a non-specific exo-enzymes produced mainly by bacteria (Alori et al., 2017). In addition to their positive contribution to the enhancement of P bioavailability, PSB-mediating soil P availability possess other worthy attributes of agronomic interests, including production of plant hormones, enhancing the ability to resist biotic and abiotic stresses through producing specific (e.g., antifungal) compounds, and the regulation of key metabolic pathways (Sharma et al., 2013).
All these worthy benefits of PSB contributed, in integration with P foliar application, to the high performance (growth and yield; Tables 3 and 4) and P content of Phaseolus vulgaris plants on calcareous soil (Rady et al., 2020). This high plant performance and P content is attributed to the increase in leaf photosynthetic pigments, photosynthesis efficiency and net photosynthesis rate of common bean plants occurred by treatments providing P to the plant, especially the integrative PSB+NP treatment (Table 5). Leaf photosynthetic pigments such as chlorophylls (Chl) and carotenoids (Car) play key roles in light reaction of photosynthetic process in plants by capture of solar energy for CO2 fixation (Rady et al., 2020). Photosynthesis efficiency mostly relies on the solar energy absorbed by Chl molecules and performance of photosynthetic apparatus. Therefore, photosynthetic pigments are an important part of photosynthetic processes and are used to assess the relative influence of various stresses on photosynthetic properties (Netto et al., 2005; Singh et al., 2017). In the phase of light reaction of photosynthesis process, most excitation energy of PSII is converted into chemical energy [e.g., ATP and NADP(H)], which is broadly consumed in the fixation of CO2 and photorespiration coupled in the phase of dark reaction (or light-independent). However, the excess energy is dissipated as heat and a small fraction is lost as fluorescence upon de-excitation of PSII (Maxwell and Johnson, 2000). Because P is involved in the synthesis of phospholipids forming plasma membranes, the bioavailability of P is very important for stability of thylakoid membrane that is necessary for biosynthesis and positioning of Chl molecules, which may affect the overall yield of photosynthesis. Therefore, supplying plants with P preserved the contents of leaf photosynthetic pigments (Table 5), which had a positive correlation with common bean yields (Table 4). In addition, maintaining the efficiency of PSII (Fv/Fm) function (Table 5) has contributed, along with the beneficial effects of P (Rady et al., 2020), to common bean plant performance under calcareous conditions. The P-provided plants has stabilized cell membranes in terms of low malondialdehyde (MDA) content (index of lipid peroxidation) and electrolyte leakage; EL (Table 6). This result seems to be due to the ability of plants supplied with P to cope with stress factors as found in this study.
Supplying common bean plant with P (especially by the integrative PSB+NP treatment) showed better returns (growth and yield), probably due to the improved translocation of photosynthesized assimilates from leaf to pod due to the longer photosynthetic duration under stress of this study (the calcareous state). Phaseolus vulgaris plants provided with P have stay-green feature (data not shown), which are approved to increase the period of seed filling under stress. This outcome is attributed to the ability of plants to optimize their water content in term of increased leaf relative water content (Table 6). This enabled plants to perform well with respect to meristems activities and cell expansions as a result of maintaining sufficient amount of water against the stress under study (high CaCO3) due to the worthy increase in the contents of osmoprotectants (Rady et al., 2018 & 2020).
P induced increase in Phaseolus vulgaris growth and production (Tables 3 and 4) was associated with a worthy increase in chlorophyll biosynthesis, photosynthetic efficiency, and net photosynthesis rate (Table 5) and a worthy decrease in oxidative stress biomarkers (in terms of decreased H2O2, and O2•‒ accumulations), thus membrane integrity (in terms of minimum MDA accumulation and EL; Table 6). After supplying Phaseolus vulgaris plants with P, the efficiency of photosynthesis was increased with raising the ameliorative effects on biosynthesis, photosynthetic efficiency, and net photosynthesis rate along with the reduction in oxidative stress biomarkers (Mohamed et al., 2006,Ghallab et al.,2007, Rady et al., 2019,Rady et al., 2020).
Results of this study suggest that the improvement in photosynthetic efficiency due to the increased P or its solubilization (due to P foliar spray for plant or PSB inoculation of soil) may largely depend on the protected functioning of the light reaction of photosynthesis that functionally coincided with PSII and PSI enzymes. In addition, photosystems functioning is associated with the trans-thylakoid proton gradient generation, creating a pH difference to regulate electron transfer to the PSI. This electron transfer has been notified under the direct control of the proton gradient regulation-5 (PGR5) protein (Tikkanen et al., 2015). The higher PSII activity in P provided plants is likely to prevent the formation of singlet oxygen (1O2) and thus protects the structure of chloroplasts from the oxidative damage resulted from the calcareous conditions (Rady et al., 2020). Supplying Phaseolus vulgaris plants with P (especially by the integrative PSB+NP treatment) was efficacious in preserving and safeguarding the assimilation rate of CO2 and net photosynthesis rate (Rady et al., 2020), thus improving growth and yield performances (Tables 3 and 4).
The reductions of H2O2 and O2•‒ accumulations, lipid peroxidation (MDA content), and electrolyte leakage (EL) were observed in P-provided plants under high CaCO3 stress (Table 6). Supplying plants with P significantly minimized membrane EL and MDA content, and thus improved membrane integrity, which can be attributed to the positive effect of P in maintenance of plant water status and low rates of peroxidation (Table 6).
Conclusions:
Based on the study results, it can be concluded that soil inoculation with phosphate-solubilizing bacteria in integration with foliar spray using phosphorus in nano-particles has improved growth and productivity of Phaseolus vulgaris plant under high carbonate (CaCO3; calcareous state) stress by up-regulation of plant water status and reducing the levels of oxidative stress biomarkers. The decrease in oxidative stress induced by reactive oxygen species production coincided with a decrease in lipid peroxidation (determined as malondialdehyde content) in phosphorus-provided plants led to maintenance of cellular functioning and higher photoprotection. This has resulted in boosted protection of leaf chlorophylls and carotenoids. Phosphorus-mediated growth improvement under high CaCO3 stress may be attributed to the enhancement of reactive oxygen species detoxification system by the interplaying among different signaling components. All these observations point to the appropriateness of the integrative phosphate-solubilizing bacteria + phosphorus in nano-particles to exploit the genetic potential of Phaseolus vulgaris plant under high carbonate stress. However, more systematic studies are needed to explain the mechanisms of plants taking up phosphorus in nano-particles as a nutrient source and why phosphorus in nano-particles performed better over the conventional phosphate fertilizer; mono-ammonium phosphate or calcium superphosphate in improving plant growth and yield. Therefore, future investigations in this tendency can be helpful.