Agricultural Research Pesticides and Biofertilizers
OPEN ACCESS | Volume 6 - Issue 1 - 2026
ISSN No: 2994-0109 | Journal DOI: 10.61148/2994-0109/ARPB
Aden Giro *, Teshale Sori, Dereje Tesfaye
(DVM, MVSc in Veterinary Epidemiology) Addis Ababa University, College of Veterinary Medicine and Agriculture.
*Corresponding Author: Aden Giro, (DVM, MVSc in Veterinary Epidemiology) Addis Ababa University, College of Veterinary Medicine and Agriculture.
Received: October 19, 2021
Accepted: December 14, 2021
Published: January 06, 2022
Citation: Aden Giro, Teshale Sori and Dereje Tesfaye. (2022) “Sero-epidemiological Investigations of Camel Brucellosis and Community Perception in Selected Districts of Borana Zone, Southern Oromia, Ethiopia.”, Journal of Agricultural Research Pesticides and Biofertilizers, 4(1); DOI:http;//doi.org/01.2022/1.1044.
Copyright: © 2022 Aden Giro. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Understanding the epidemiology and natural history of camel brucellosis is crucial for control. A cross-sectional study was conducted in two selected districts of Borana Pastoral setting, Southern Ethiopia from November 2020 to April 2021 to estimate sero-prevalence and assess associated risk factors of camel brucellosis. A total of 315 blood samples were collected from camels older than 6 months in Arero and Elwoye districts. The collected serum samples were screened using Rose Bengal plate test and positive samples were confirmed using indirect enzyme-linked immunosorbent assay. The association between potential risk factors and sero-prevalence was computed using multivariable logistic regression and chi-square tests. Out of the total of 315 serum samples screened by Rose Bengal plate test 29 (9.21 %; 95 % CI: 6.25 – 12.95) were positive for brucellosis, of which 9 (2.86 %; 95 % CI: 13.15 – 53.54) were confirmed to be positive using indirect enzyme-linked immunosorbent assay. The statistical analysis showed that female camels which had history of abortion were more likely to be seropsotive than those which did not have abortion history (χ2=5.51; p=0.014 and OR=6.2; 95 % CI=1.08 -35.86). Camels tested from large herd size were more at risk of acquiring brucellosis than those from small herd size (χ2=9.02; p=0.0027and OR=17.04; 95% CI=1.77-164.04). The prevalence was higher (3.17 %; 95 % CI: 0.87 – 7.93) in Elwoye district than in Arero (2.65 %; 95 % CI: 0.86 – 6.07) although the difference was not statistically significant. The results of questionnaires revealed that 33 (73.33 %) of the respondents knew the brucellosis. Most of the animal herders had greater knowledge about the brucellosis than other participants of the study, which was statistically significant (P<0.05). The major signs of brucellosis recognized by the pastoralists include abortion, retain placenta and stillbirth with 100%, 81.82% and 66.67%, respectively. The majority of the pastoralists interviewed (27; 81.82 %) were not aware about brucellosis being transmitted from domestic animals to humans. Although the prevalence of brucellosis observed in this in camels is low, the lack of control and prevention programs could make it a public health threat for the pastoral community.
Camels (Dromedaries) are important livestock species adapted to hot and arid environments prominently due to its unique anatomical, physiological and behavioral characteristics. It highly contributing to food security and social stability in the pastoral areas of Africa and The Middle East. Camels not only serve the community by providing food and darft power but also they are used to fetch water and other resources used for other livestock species during harsh conditions. The optimal utilization and the development of camel production is, however, hampered by different technical and non-technical constraints including infectious diseases [1]. Brucellosis is one of infectious Camel disease caused by Brucella abortu (B. abortus), Brucella melitensis (B. melitensis), Brucella ovis (B. ovis) and Brucella suis (B. suis) with considerable public health and economic importance. Geographical distribution of brucellosis occurs more frequently in countries with poorly standardized animal and public health services [2]. Camel brucellosis is endemic in countries of the Mediterranean basin, Middle East, Central Asia, horn of African countries such as Ethiopia, Eritrea, Somalia and Sudan [3] Where extensive traditional production with minimal veterinary services. In these areas brucellosis has been reported in many domestic animal species including human beings [4].
The occurrence of brucellosis can be in any season of a year but the epidemic peak is mostly associated with delivery and abortion in animals [2]. Poor management and large herd size contribute to high prevalence of brucellosis. Increase in herd size increase the chances of contact between animals leading to infection particularly during calving or abortion [5]. This is often the practice adopted in pastoral areas where large number of animals of various age and species are reared together. Mixing of camels with other domestic animals during the time of migration, at watering time, in communal rangeland or at night enclosure can play role in the transmission of the disease from infected species to camels [6]. Transmission of brucellosis in animals occurs mainly through ingestion of food or water contaminated by infected uterine discharges, aborted fetuses or fetal membranes and even through licking the genital of diseased animals. In addition, infected males can also spread the infection among females through natural mating and artificial insemination [7]. The most common clinical manifestation of brucellosis in Camel is Abortion in pregnant Camels infected with Brucella organism’s and non-Pregnant developed only mild, transient clinical symptoms including reduced appetite, slight lameness and bilateral lacrimation [8], stillbirth or a weak, non- viable calf, retain placenta, placentitis, uterine infections, fetal mummification and death, delayed maturity and infertility. Other conditions caused by the disease in male camel were Orchitis, epididymitis, arthritis and hygroma have also been associated with brucellosis [4].
Bacterial isolation is the gold standard in diagnosis of brucellosis, which relevant under epidemiological point of view. It requires long cultivation periods and great care during handling any material containing Brucella organisms [9]. A serological test is another test which frequently used to diagnose camel brucellosis, which include RBPT (Rose Bengal Plate Test), CFT (Complement fixation test), ELISA (enzyme-linked immunosorbent assay), FPA (Fluorescence Polarization Assay) and SAT (Serum agglutination test [10]. Different serological tests combination can increase diagnostic efficacy of tests [3]. Generally brucellosis cause significant loss of productivity through low herd fertility as a result of abortions, sterility, late first calving age, long calving interval time and comparatively low milk production [9]. The costs associated with medical care of Brucella infected humans and the duration of time the infected people are out of work account for financial losses [11]. The disease can also have an impact on export and import of animals constraining livestock trade and is an impediment to free animal movement [12]. In Ethiopia, brucellosis is endemic and highly prevalent in cattle, camels and small ruminants in pastoral and agro-pastoral areas [5]. Camel brucellosis has been reported from pastoral areas, with prevalence ranging between 0.73 to 11.9% when RBPT was used for screening and 0.53 to 9.6% using CFT [13]. The differences in prevalence is hypothesized to be associated with different environmental and management conditions [14].
The ability of the camel to survive in harsh areas of the world, its endurance in prolonged drought, and above all its high potential to convert the scanty resources of the desert into milk and meat makes them more important to the pastoralists. Camels are versatile animal species in ensuring food security and fulfilling the livelihood priorities of pastoral households [15]. Its production would only effective in understood and improved factor affect productivity and health burden. Since camels are becoming important livestock species in the pastoral areas where millions of people inhabit, understanding epidemiology and natural history of brucellosis is crucial. Therefore, this study was conducted to assess the community knowledge about camel brucellosis and estimate its prevalence in Borana zone.
The specific objective is:
2. Literature Review:
2.1. Brucella Organisms:
2.1.1. Historical prospective of brucella organisms:
Brucella is an organism’s that has very old history of detection in carbonized cheese from the Roman era [7]. Several synonyms of brucellosis have been known like Malta fever, undulant fever, Rock of Gibraltar fever and Bang’s disease. In 1884, Dr. Bruce was able to differentiate between brucellosis (Malta fever) and typhoid outbreaks affected in Malta. Three years later, he isolated the causative agent of Malta fever and named the bacterium Micrococcus melitensis [2]. In 1897, Dr. Bang studied the disease in Denmark and could isolate B. abortus strains from aborted cattle. He noticed that the pathogen can also infect sheep, goat and horses; the disease became known as Bang’s disease. Later in 1918, Evans detect connection between animal and human cases after he isolated an organism from human aborted fetus which was closely related to Bruces‘s organism. In the year 1938, it was possible to differentiate among the caprine, bovine and swine forms of Undulant fever caused by B. melitensis, B. abortus and B. suis, respectively [7]. Camel brucellosis has not received proper attention from researchers and scientists. Brucellosis was reported in camels as early as in 1931 by Solonitsiun in Russia then the disease has been reported from all camel-keeping countries. Camel brucellosis is a wide spread disease in camel rearing regions of the world such as middle East and the Arabian Gulf, parts of Africa, and Latin America with the exception of Australia [3].
2.1.2. Etiology of brucellosis:
Brucellosis is a disease affecting a wide range of animal species including human beings, and caused by non-motile, aerobic, gram negative belonging to the cocobacilli genus of Brucella. The genus Brucella consists of at least six species, designated on the basis of host preference, antigenic [16] and biochemical characteristics as B. melitensis (goats, sheep and camel), B. abortus (cattle and camel), Brucella suis (pigs), Brucella canis (dogs), Brucella ovis (sheep) and Brucella neotomae (wood rats), Brucella pinnepedialis, Brucella ceti (marine species) [17]. In humans, B. melitensis, B. abortus, B. suis and B. canis are potential agents of brucellosis, B. melitensis being the most virulent species for humans [18]. Major causative agents of brucellosis in camels are B. abortus, B. melitensis, B. ovis and B. suis (Table 1). Frequently B. abortus and B. melitensis are isolated from milk, aborted fetus and vaginal swabs of diseased of camels [16]. Even though camels are not known to be the primary hosts of Brucella, they are susceptible to both B. abortus and B. melitensis consequently, the infection depends upon the infection rate in primary hosts being in contact with them [3].
|
Country |
Brucella species |
Specimen |
References |
|
Jordan |
B. melitensis biotype 3 |
Aborted foetus, milk |
[19] |
|
Iran |
B. melitensis biotype 1 B. abortus biotype 1 |
Lymph nodes Blood |
[16] |
|
Yemen |
B melitensis |
Vaginal swabs & blood |
[20] |
|
Libya |
B.melitensis biotype 1 |
Milk, aborted foetus, vaginal swab |
[21] |
|
Egypt |
B. melitensis biotype 3 B. abortus biotype 1 B. suis biotype 1 |
Milk |
[22] |
Table 1: Brucella species infecting camels reported from different countries of the world.
2.2. Epidemiology of Camel Brucellosis:
2.2.1. Geographical distribution:
Brucellosis is a worldwide bacterial disease affecting both animals and humans which subsequently causes serious human health hazards and economic loss. The geographical distribution of brucellosis shows that it is common in countries with poorly standardized animal and public health programed [2]. Though it has been eradicated from many developed countries like Australia, Canada, Israel, Japan, New Zealand and Europe), it remains an uncontrolled problem in regions of high endemicity such as Africa, Mediterranean, Middle East, and parts of Asia and Latin America [23]. Camel brucellosis is a wide area distributed disease were camel raring are being practiced. It is endemic in countries of the Mediterranean basin, Middle East, Central Asia, horn of African countries such as Ethiopia, Eritrea, Somalia and Sudan [3]. The prevalence of camel brucellosis reported from different countries is presented in Table 2.
|
Country |
Prevalence % |
Lab Test |
Reference |
|
Pakistan |
21% 21% 13% |
RBPT SAT c-ELISA |
[24] |
|
Libya |
5.7% |
CFT |
[25] |
|
Oman |
1.5% |
c-ELISA |
[14] |
|
Kenya |
2% 10.5% |
RBPT SAT |
[26] |
|
Egypt |
4.17% 3.73% |
m-RBPT c-ELISA |
[22] |
|
Eritrea |
3.1% |
CFT |
[27] |
|
Iran |
13 % |
PCR |
[16] |
|
Iraq |
3.03% |
RBPT/ 2ME |
[28] |
|
Sudan |
5.8% 5% |
RBPT c-ELISA |
[9] |
|
India |
8.9% 4.9% |
RBPT ELISA |
[29] |
|
Nigeria |
11.2% 10.5% |
RBPT SAT |
[30] |
|
Somalia |
1.7% 3.9% |
RBPT c-ELISA |
[31] |
|
Yemen |
5.1% |
MRT |
[20] |
Table 2: Summary of occurrence of camel brucellosis in the world
Brucellosis can affect almost all animal species including human beings, and cross transmission can occur between cattle, sheep, goats, camels and other species. It causes significant reproductive losses in sexually mature animals [4]. Susceptibility to infection depends on pregnancy status, age, sex, and breed of the animals. Sexually matured animals are more prone to Brucella infection than sexually immature animals of either sex. On the other hand, it is also true that younger animals tend to be more resistant to infection and frequently clear an established infection, although latent infections can occur [11]. This may be due to the fact that sex hormones and erythritol, which stimulate the growth and multiplication of Brucella organisms, tend to increase in concentration with age and sexual maturity [32]. Occurrence of brucellosis is not seasonal but the epidemic peak occurs season is associated with delivery and abortion in animals [2]. After reaching sexual maturity, the state of pregnancy has a greater influence on the degree of susceptibility. In pregnant camels, the bacteria localize in the placenta and are most abundant in abortion material (up to 1013 bacteria) including the fetal stomach, vaginal discharge and colostrum [33]. parturition in camels is occurred in a laying or standing position without extra help, they may deliver or abort on the pasture and the aborted material may spread over a wide area of the pasture by stray dogs and foxes [3].
Poor management and large herd size contribute to high prevalence rate of brucellosis. Increases in herd size increase the chances of contact between animals which leading to infection particularly during calving or abortion [5]. Placentophagy with camels as a noted exception, which may contribute to the transmit of Brucella organism [18]. Camel herd kept in close contact with other domestic animals during the time of migration, at watering time or at night enclosure can also play the transmission of the disease from infected animals to healthy ones [6]. Close contact between infected and susceptible camels, and sharing the same watering points and pastures with other livestock promotes the spread of diseases [8]. Survival of the organisms in the environment is enhanced by cool temperatures and humidity however it can also survive in a hot desert environment [34]. Under appropriate conditions, Brucella organisms can survive in the environment for prolonged periods. Their ability to withstand inactivation under natural conditions is relatively high compared with most other groups of non sporing pathogenic bacteria. B. abortus survival outside the host is largely dependent on environmental conditions. The pathogen may survive in aborted fetus in the shade for up to eight months, for two to three months in wet soil, one to two months in dry soil, three to four months in faeces, and eight months in liquid manure [35].
Brucella has ability to adapt to the environmental conditions in intracellular replication including low levels of nutrients and oxygen, acidic pH and reactive oxygen intermediates. Inside the cells, Brucella has the ability to interfere with intracellular trafficking, preventing fusion of the Brucella containing microphages with lysosome markers, and directing the vacuole toward a compartment that has rough endoplasmic reticulum, which is highly permissive to intracellular replication of Brucella [3]. These endoplasmic reticulum-associated compartments are the niche for intracellular replication of Brucella in macrophages, epithelial cell lines and placental trophoblasts. Once inside this compartment, the bacteria can establish chronic infection [36].
Brucellosis is transmitted horizontally under normal conditions. Domestic and wild animals can contract brucellosis through direct contact with infected animals and their excreta. The primary shedding routes of organisms is uterine fluids and placenta expelled from infected animals [6]. Natural infection in animals occurs mainly through ingestion of feed or water contaminated by uterine discharges, aborted fetuses or fetal membranes and even through licking the genitalia of diseased animals. In addition, infected males can also spread the infection among females through natural mating and artificial insemination. Brucella can pass through intact or injured skin and mucous membranes [7]. Brucellosis is transmissible from animals to humans through contaminated milk, raw milk products, meat or direct contact with infected animal blood, placenta, fetuses or uterine secretions, handling infected animal fetus and placenta. Person to person transmission is rare, but it being transmitted by close personal or sexual contact, blood donation, tissue transplantation and Bone marrow transfer [18].
2.3. Pathogenesis:
Brucella infection depends on natural resistance of the animal to the organisms, virulence of the Brucella specious and exposure dose. Organisms enter animal hosts through skin abrasions, reproductive tracts, gastrointestinal tract, respiratory tract and conjunctiva. In the alimentary tract the epithelium covering the ileal Peyer’s patches are sites of entry [38]. Brucella penetrates the mucosal epithelium and transported as free bacteria or engulfed by phagocytic cells. After penetration and localized to regional lymph nodes it proliferates, disseminate haemogenously and localize in the reticulo endothelial [39]. Various mechanisms employed by Brucella organisms to survive inside the phagocytic cells is inhibiting phagolysosome fusion, blocking bactericidal action of phagocytes and suppressing the myeloperoxidase H2O2 halide system [9]. They are taken up in phagosomes, re-main viable by suppressing phagosome-lysosome fusion, and inhibit apoptosis of host cells. They multiply in vacuoles within the endoplasmic reticulum and spread to various organs, particularly into the cells of the reticulo endothelial system, liver, urogenital tract, spleen and skeletal muscle where they give rise to granulocytic inflammation with or without necrosis or caseation [38].
Organisms spread through the hema-togenous route reaches the placenta and finally to the fetus. The preferential localization to the reproductive tract of the pregnant animal is due to the presence of the allantoic fluid factors that would stimulate the growth of Brucella. Four carbon alcohols (Erythritol) is one of the factors which elevated in the placenta and fetal fluid from end of second trimester of gestation. An initial localization within placentome adjacent to chorioallantoic membrane results in rupture of the cells and ulceration of the membrane. The damage to placental tissue together with fetal infection and fetal stress inducing maternal hormonal changes that cause abortion [38].
2.4. Clinical Signs of Brucellosis:
Clinical symptoms variation of brucellosis is typical consequence of level of immunity, environmental influences, age, pregnancy status and virulence of the pathogen. Camel brucellosis is characterized by Abortion in pregnant Camels infected with Brucella organism’s and non-Pregnant developed only mild, transient clinical symptoms including reduced appetite, slight lameness and bilateral lacrimation [8], retain placenta, placentitis, uterine infections, fetal mummification and death, delayed maturity and infertility. Other conditions caused by the disease in male camel were Orchitis, epididymitis, arthritis and hygroma have also been associated with brucellosis [4]. Human brucellosis is a disease that may have variable clinical sign after exposure to the bacteria; clinical manifestations may appear within five to sixty days. Infected patients with acute disease consisting of general symptoms, such as fever, malaise, sweats and lymphadenopathy and hepato splenomegaly [8]. Chronic brucellosis is more severe form of the disease that can be associated with osteoarticular signs including spondylitis, arthritis and osteomyelitis, or genitourinary infection, such as orchitis, epididymitis, glomerulonephritis and kidney abscesses. Life-threatening complications comprise, in descending order of frequency, neuro brucellosis, liver abscesses, and endocarditis [36].
2.5. Diagnosis of Brucellosis:
The diagnosis of brucellosis can be challenging and is frequently delayed or missed because the clinical picture may mimic other infectious and non-infectious conditions. Thus, It is very difficult to make a diagnosis based on clinical signs despite abortions in the third trimester being indicative of brucellosis; this is because other infectious diseases such as leptospirosis, Rift valley fever and Listeriosis can also cause abortion storms [37].
The microorganism can be identified by microscopic examination of stained smear from vaginal dis- charges, placenta, colostrum, fetal stomach fluid or of the aborting cow’s lochia, and the abomasum of the aborted fetus using the modified Ziehl-Neelsen (MZN) stain. Impression smears may be taken from freshly cut and blotted tissue surfaces, e.g. cotyle- dons, by firmly pressing the slide surface against the tissue. Allow to air dry and heat fix smears [38]. Brucella is not a true acid- fast bacillus but show resistant to decolorization by week acids. They seem like short rods or coccobacilli, mostly arranged singly but occasionally in pairs or small groups. They appear as coccobacilli or short rods, usually arranged individually but sometimes in pairs or small groups [40]. However, morphologically related micro-organisms, such as Chlamydophila abortus, Chlamydia psittaci and Coxiella burnetti can mislead the diagnosis because of their superficial similarity. Accordingly, the isolation of B. melitensis on appropriate culture media such as Farrell’s selective media is recommended for an accurate diagnosis [41].
The gold standard in the diagnosis of brucellosis is bacterial isolation (culture), which relevant under epidemiological point of view. It requires long cultivation periods and great care during handling any material containing Brucella organisms. Brucella Spp. is classified as a Biosafety level 3 organism, which manipulation should be performed in biosafety level-3 laboratories [9]. Brucellosis is one of the most common accidental laboratory infections, particularly in research laboratories. All Brucella strains are relatively slow growing and use of a selective medium, e.g. Farrell’s medium because of specimens from which isolations best are heavily contaminated [38]. Specimens which used for Brucella isolation include milk (colostrum or milk within a week of calving) vaginal swabs; semen and aborted fetus are useful for diagnosis of organisms at ante mortem. Samples collected at necropsy include spleen, udder, pieces of uterus and testicular tissue, fetal stomach fluid, supra mammary lymph nodes (chronic and latent infections) and retropharyngeal (early infections) are preferred, but iliac, pre scapular and parotid may be used. If serological reactions are thought to be caused by S19 vaccine strain then it is important to collect pre-scapular lymph nodes as well [9].
Demonstration of the bacteria is by staining with Gram-negative stain or modified-Zeihl Neelsen staining florescent antibody test and polymerase chain reaction methods for Brucella species identification[9]. B. Spp. colonies are elevated, transparent, convex with intact borders, smooth, and a brilliant surface. The colonies have a honey color under transmitted light. Optimal temperature for culture is 37 °C whereas optimal pH ranges from 6.6 to 7.4. Some Brucella spp. requires CO2 for growth. Typical colonies appears 2 to 30 days of incubation, but a culture can only be considered negative when there are no colonies appears 2 to 3 weeks of incubation [38].
Serological tests frequently used to diagnose camel brucellosis include RBPT, CFT, ELISA (competitive and indirect), FPA and SAT. Different serological tests combination can increase diagnostic efficacy, although none of the serological tests can differentiate Brucella species. False-positive or unspecific reactions with various other bacterial species may occur [3]. All tests have limitations concerning specificity and sensitivity, especially when testing individual animals [10]. RBPT is known as the buffered Brucella antigen tests which rely on the presence of antibodies against antigen of Brucella in the serum. The principle is based on the ability of IgM antibodies bind to antigen is markedly reduced at a low pH [42]. It is very sensitive and suitable test for screening herds for brucellosis, but false positive results due to vaccination with B. abortus strain 19 vaccine or cross reactions with other bacteria. RBPT may not be absolutely reliable among commonly used serological diagnostic tests for brucellosis. RBPT detected antibody in the sera of fifty percent of the animals suspected for brucellosis [3].
Competitive ELISA (c-ELISA) is the most sensitive test for the diagnosis of brucellosis. Doubtful or positive samples with RBPT were further confirmed by c-ELISA [33]. c-ELISA using a commercial DNA extraction kit according to the manufacturer’s protocol. Gene amplification was performed in a thermal cycler. c-ELISA, using S-LPS or OPS as antigens, are used for brucellosis serology. Different antiglobulin-enzyme conjugates, substrate/ chromogens and antigens are prepared from different smooth Brucella strains. The c-ELISA uses a monoclonal antibody specific for one of the epitopes of the Brucella spp. OPS antigens have higher specificity, but slightly lower sensitivity than i-ELISA. This assay is an excellent confirmatory assay for the diagnosis of brucellosis in most mammalian species [43]. Indirect enzyme linked immunusorbent assay (i-ELISA) is most commonly used system depends on enzymes for detection and consists of smooth Lipopolysaccharide (S-LPS) preparation attached to a polystyrene matrix in 96 well plates. i-ELISA s has high sensitivity, but the specificity can be rather low. Commercial kits using whole cell, S-LPS or the O-polysaccharide (OPS) as antigens have been validated and results obtained from different assays are not always comparable [44]. CFT allows the detection of anti-Brucella antibodies that are able to activate complement. Many authors regarded the CFT as being the most sensitive and specific test for brucellosis diagnosis because CFT antibodies remain in the serum for longer period of time than SAT antibodies. On the contrary, some authors disclosed that this test is not highly sensitive but shows an excellent specificity. In the recent year CFT is progressively being replace by ELISAs since it is difficult to be standardized. Nevertheless, CFT is a “prescribed test for trade” by the OIE [3].
FPA is simple and the rate of rotation of a molecule in solution is inversely proportional to its size. A small molecule will rotate rapidly while larger molecules rotate more slowly. By attaching a fluorescing molecule to an antigen molecule, the rate of rotation can be measured using polarized light. The result is a measurement of the time it takes the molecule to rotate through a given angle. In the case of brucellosis serology, small molecular weight subunit of O-polysacharide (OPS) is labeled with fluorescein isothiocyanate and used as the antigen. When testing serum, blood or milk, if antibody to the OPS is present in the samples tested, the rate of rotation of the labeled antigen will be reduced. The rate of reduction is proportional to the amount of antibody present [45]. The SAT is simple, cheap and lack of sensitivity and specificity mean that it should only be used in the absence of alternative techniques. It has been used extensively for brucellosis diagnosis. A suspension of Brucella possessing active antigen will agglutinate when exposed to homologous Brucella antibody. This agglutination forms clumps of bacteria which become macroscopically visible. SAT is used to detect brucellosis by measuring agglutinating antibodies of the IgM, IgG 1, IgG 2, and IgA types. The SAT can be used to detect acute infections, as antibodies of the IgM type usually appear first after infection and are more reactive in the SAT than antibodies of the IgG 1and IgG 2 types. However, because the SAT may yield both false negative or false positive results it effectively detects brucellosis only on a herd basis [46].
Milk ring test (MRT) is serum agglutinations test used to identify the accurateness of antibodies against Brucella spp. in milk. It suggested as a screening test to check Brucellosis is bulk tank milk. MRT is done by cream or whole milk [41]. Hematoxylin Brucella stained cells are added to milk and incubated for the reaction. MRT detects the IgM and IgA immunoglobulin. False adverse reaction in abnormal milk is due to mastitis, milk from the late lactation due to the presence of colostrum. Low concentration of lacteal antibodies or lacking fat, clustering, and factors in milk may also cause a false-negative result. Despite all these problems, the milk ring test is very successful, it is the method of choice in dairy herds, and it is a low-cost screening test as compared to other [40].
Molecular techniques are important tools for diagnosis and epidemiologic studies, providing relevant information for identification of species and biotypes of Brucella spp. allowing differentiation between virulent and vaccine strains. Molecular detection of Brucella spp. can be done directly on clinical samples without previous isolation of the organism. In addition, these techniques can be used to complement results obtained from phenotypic tests. Despite the high degree of DNA homology within the genus Brucella, several molecular methods, including PCR, PCR restriction fragment length polymorphism (RFLP) and Southern blot, have been developed that allow, to a certain extent, differentiation between Brucella species and some of their biovars [47].
PCR based techniques have been developed in recent years and are in use as alternative diagnostic tests for brucellosis. They are based on the detection of specific sequences of Brucella spp. DNA in clinical samples. PCR techniques have lower diagnostic sensitivity and higher specificity than culture methods hence best results are obtained when the two are combined [37].
Allergic skin test (AST) is an allergic test that measures cellular immune response which has been used by some researchers, particularly on Bactrian camels in the former USSR. AST based on a delayed type hypersensitivity reaction with a maximum sensitivity at 72 hours post inoculation increase in the thickness of skin at the site of inoculation. The antigen does not induce animal’s immune system and not interfere in the diagnosis of the disease and decrease the of false-positive reactions. The skin test is highly specific and weak sensitivity. Thus, it is often suggested for use at the herd level as a positive test in unvaccinated animals [33 and 40].
The Mercaptoethanol Test (2-MET) are two forms that use either 2-mercaptoethanol or dithiothreitol. Dithiothreitol has recommended, because of the toxicity of 2-mercaptoethanol. The disulfide of IgM is being condensed to the manometric molecule and unable to agglutination essentially calculate IgG unable to agglutinate. However, IgG can also be decreased in the procedure, providing false-negative results. Though in general, reduction of IgM increases specificity. The test not suggested for the global trade due to not eradication vaccinal antibodies. The 2-MET is, however, used prominently for national control and eradication programs [41]
Animal inoculation may be either through abraded skin or subcutaneously in guinea-pigs or, preferably, through the digestive tract or nasal (aerosol) intravenously, or intra peritoneal routes in mice. The spleen of mice is cultured seven days after inoculation, while serum samples of guinea pigs are subjected to specific tests three and six weeks after inoculation. It is noteworthy however, that in laboratory animal gastric acid can interfere with the infectivity of Brucella [41].
2.6. Host Protective Immune Response:
Infection with Brucella usually results in the induction of both humoral and cell-mediated immune responses. The magnitude and duration of these responses can be affected by many factors including virulence of the infecting strain, size of inoculum, age, sex, pregnancy, species, and immune status of the [48].
humoral immune response plays an important role in immunity to provide protection. Protective mechanisms of humoral immunity against intracellular pathogens may rely on combination of factors that include antibody isotype and function. Antibodies have a protective role against reinfection with Brucella but their role in protection against primary infection is less explicit. Innate or alternate immuno-protective mechanisms that precede development of humoral immunity are sufficient to control primary infection and the synergistic and inhibitory contributions of specific antibodies need to be further explored [49].
IgG1, IgG2, IgM, and IgA are the immunoglobulin isotypes present in animal serum. The first immunoglobulin produced after an initial heavy infection or strain 19 immunization is IgM. This can usually be detected in the first or second week following the initial antigenic stimulus but is soon followed by IgG antibody. IgG1 immunoglobulin is the most abundant in serum and exceeds the concentration of IgG2. The magnitude and duration of the antibody response following immunization is directly related to the age at immunization and the number of organisms administered. Following immunization with the standard dose of strain 19 during calf hood, IgG antibody concentrations usually decline to diagnostically insignificant levels over 3 - 6 months [11].
Brucellae are facultative intracellular bacteria that survive and replicate in both phagocytic and non-phagocytic cells. Due to the chronic nature of many diseases caused by intracellular pathogens, an effective adaptive response is necessary to control disease. Several components of the immune system contribute to protection against intracellular pathogens. Cell mediated immune response helps to remove the infection and creates memory component to that specific antigen in the host, which is an essential property in long lasting vaccination response [50] critical for protection against Brucella and other intracellular pathogens such as Chlamydia, Francisella, and Mycobacterium [49].
Phagocytic cell process and presenting antigens to initiate T-cell responses which play a major role in acquired specific resistance to intracellular bacteria determines the resolution of infection [51]. Macrophages and T-cells play crucial roles in protection. Helper T-cell mediated protection is primarily associated with a Th1 T-cell response and persistence (chronic brucellosis) with a Th2 response [49]. Macrophage-derived cytokines which are interleukin 1 (IL-1), IL-12, and tumor necrosis factor alpha (TNF- ) plays important role in control of early Brucella spp. infection by IFN- pathway [50]. Immune response can control Brucella infection by IFN- activates the bactericidal function on Brucella residing in the macrophages in order to prevent the intracellular survival and IFN- is produced by CD4+, CD8+, and T cells cytotoxicity mechanism of CD8+ and T cells destroys Brucella infected macrophages. Th1-mediated antibody isotypes, such as IgG2a and IgG3 engulf the pathogen to promote phagocytosis and degradative endocytic compartments [50]. Cytokines are likely to exert maximum effect early in infection and balance enhancing immunity and exacerbating disease. The combined transfer of immune serum and cells has given better protection than that provided by serum or cells alone given prior to the challenge [51].
2.7. Importance of Camel Brucellosis:
2.7.1. Public health importance:
Brucellosis is an important zoonotic disease that has been shown to cause human ailments for over one and half centuries. It has been known to be caused by B. melitensis, B. abortus, B. suis and occasionally by B. canis [11]. It is the second most important zoonotic disease after rabies which is more severe in human beings than domestic animals. Brucellosis is transmissible from animals to humans through contaminated milk, raw milk products, meat or direct contact with infected animal blood, placenta, fetuses or uterine secretions, handling infected animal fetus and placenta. Person to person transmission is rare, but it being transmitted by close personal or sexual contact, blood donation, tissue transplantation and Bone marrow transfer [18]. Human brucellosis is a disease that may have variable clinical sign after exposure to the bacteria; clinical manifestations may appear within five to sixty days. Infected patients with acute disease consisting of general symptoms, such as fever, malaise, sweats and lymphadenopathy and hepato splenomegaly [8]. Chronic brucellosis is more severe form of the disease that can be associated with osteoarticular signs including spondylitis, arthritis and osteomyelitis, or genitourinary infection, such as orchitis, epididymitis, glomerulonephritis and kidney abscesses. Life-threatening complications comprise, in descending order of frequency, neuro-brucellosis, liver abscesses, and endocarditis [36].
The incidence and prevalence of brucellosis in humans has been reported from various countries of the world (figure 1 and figure 2). The incidence and prevalence vary partially depending on the living standards and habits of the community. For example residents of the Wajir County in Kenya drink camel urine since they believe that it eliminates all the illness in the body but this practice contributes to the transmission of camel brucellosis [3]. It is usually considered an occupational disease for those engaged in handling infected animals, such as veterinarians, laboratory staff, farmers, and abattoir workers [25].
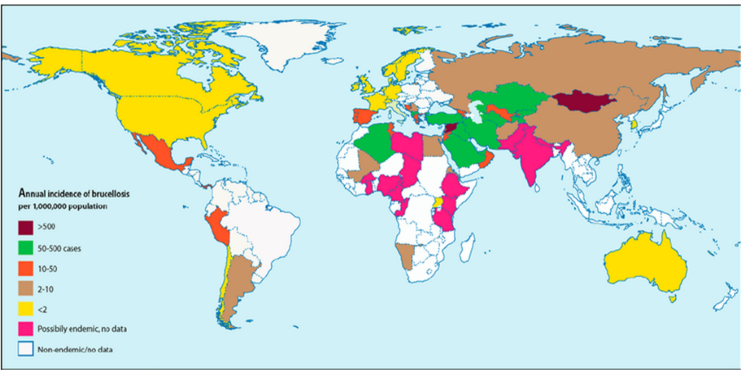
Figure 1: Incidence of human brucellosis in world
Source: [3]
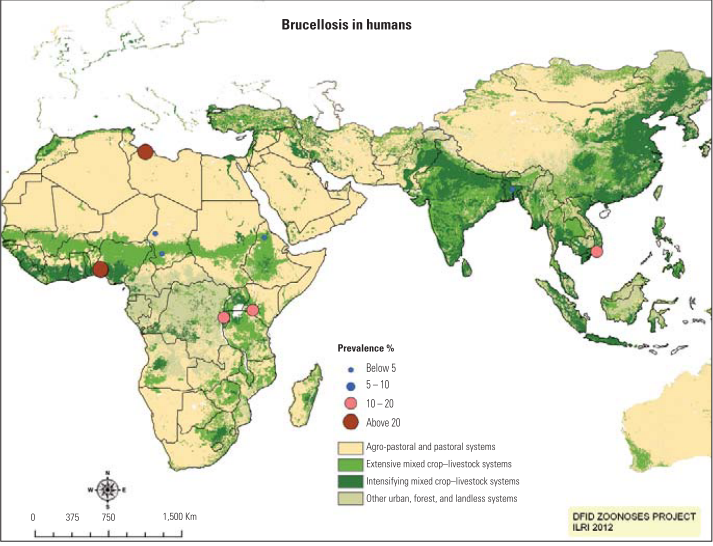
Figure 2: Prevalence of human brucellosis in world
Source:[52]
Pastoralists in Ethiopia consume raw milk, which contributes to the transmission of this disease among human and animals. more than 75% of the animal owners do not know about zoonotic Camel brucellosis [3]. The pastoral community in Ethiopia is traditionally, they consume raw animal products, sharing dwelling with their animals and poor management practices are highly prone to this disease. low awareness of the disease in general may result in high degree of transmission of the disease to human [53]. A cross-sectional study conducted by [12] in Mehoni District, Southeastern Tigray, from a total of 120 camel owners participated in the interview, about 91% (109) drank fresh raw milk regularly and 11.01% (12) of them the sero-positive. The risk of Brucella infection was found to be high (88.33%, 106) in owners with close contact to their animals (OR = 8.07, CI 95%; 0.476, 137.014) [12].
Camels are primarily the domestic animals of pastoral communities that ensure food security. They produce milk, meat, hair and hides, and serve as a draught animal for agriculture and transport people and goods [11]. Generally brucellosis cause significant loss of productivity through low herd fertility as a result of abortions, sterility, late first calving age, long calving interval time and comparatively low milk production [9]. The costs associated with medical care of Brucella infected humans and the duration of time the infected people are out of work account for financial losses [11]. The disease can also have an impact on export and import of animals constraining livestock trade and is an impediment to free animal movement [12].
2.8. Status of Camel Brucellosis in Ethiopia:
Camel population in Ethiopia is around 1.16million, out of which, 434,291 inhabits in Afar region, 353,124 in Somali region and 239,357 in Oromia region [1]. Camel production could be a profitable venture for utilizing the vast arid and semi-arid areas of Ethiopia, where other animals survive with difficulty, especially due to the recurring drought conditions. Under such environmental conditions, camels thrive and form a source of milk and meat. But, complete exploration of camels for milk and meat production would only be possible when their reproductive performance is properly understood and improved [54]. In Ethiopia, brucellosis is endemic and the disease is highly prevalent in cattle, camels and small ruminants in pastoral and agro-pastoral areas [5]. Brucellosis has been reported in camels from pastoral areas; where the prevalence was quite vary ranging between 1.9 to 12.5% for RBPT and 0.00 to 4.5% for CFT as shown in Table 3. This variation in sero-prevalence of camel brucellosis brucellosis can be attributed to different factors such as difference in animal husbandry and management systems practiced by pastoral society [55]
Study conducted by [42] in Yabello and Gomole districts of Borana Zone, revealed seroprevalence of 12.5% using RBPT for screening from which 3% of them were confirmed to be positive by using Indirect Enzyme-Linked Immunosorbent Assay (i-ELISA). A similar cross-sectional study conducted by [56] in Jigjiga and Gursum districts of Fafan Zone, Somali Regional State showed seroprevalence of (4.9%) in camels when RBPT was used to screen the sera samples. Among those positives samples by RBPT, (0.4%) of them were confirmed positive by CFT. A cross-sectional study conducted by [54] in three selected districts of Afar region of Ethiopia also revealed similar seroprevalence of camel brucellosis. These authors sampled 245 camels from the two districts and their observation revealed that 4.1% of them were confirmed to be infected by Brucella spp. by CFT. A similar scenario has been reported by Habtamu and his colleague (2015) in Mehoni district, Southeastern Tigray in which seroprevalence of 5.80% and 3.37% was observed using RBPT and CFT, respectively. Previous study investigated by [57]. However, showed lower prevalence in camels destined for export. Investigation done by [58] on seroprevalence and risk factors of brucellosis in camels brought for slaughtering at Akaki abattoir, serum samples from 201 apparently health camels were positive for brucellosis, of these, 9 (4.5%) were confirmed to be seropositive for brucellosis by CFT.
All these investigations showed that camels reared in all pastoral and few agro-pastoral areas of Ethiopia are infected with Brucella. Although the sample sizes considered and the geographical areas covered were limited, the previous results showed that brucellosis is well entrenched in camel population in the areas. This has important implication for public health particularly for those who are occupationally associated with camels.
|
Origen |
Prevalence |
Test |
Reference |
|
Akaki Abattoir |
6.5% |
RBPT |
[58] |
|
|
4.5% |
CFT |
|
|
Afar |
12.2% |
RBPT |
[54] |
|
|
4.1% |
CFT |
|
|
Tigray |
5.80% |
RBPT |
[12] |
|
|
3.37% |
CFT |
|
|
Somali |
4.9% |
RBPT |
[56] |
|
|
0.0% |
CFT |
|
|
Dire Dawa |
1.9% |
RBPT |
[59] |
|
|
1.6% |
CFT |
|
|
Borana |
12.5% |
RBPT |
[42] |
|
|
3% |
i-ELISA |
|
|
Fentale |
9.2% 9.1% |
RBPT CFT |
[60] |
|
Bale |
0.6 |
CFT |
[61] |
Previous investigations carried out showed that mixing of camels with other domestic animals during the time of migration, at watering time or at night enclosure is an important risk factor that contributes to the transmission and spread of the disease from infected animals to healthy ones [6]. The sero-prevalence of camel brucellosis has been shown to be higher in camels that have contact with cattle, sheep [54]. There are higher chances of brucellosis transmission from ruminants to dromedaries as they live in free range in promiscuity in the bush and at water points [42].
Such husbandry practices are common feature of some of the pastoral communities of Ethiopia. For example, there is free commingling of camels with ruminants in Borana pastoral areas. This might have contributed to the occurrence of camal brucellosis in the area. Studies also revealed that herds with larger size (>50 camels) had higher prevalence (36.84%) than medium (15.38%) and small sizes. Brucella seropostivity increased with large herd size while the chances of contact between animals’ increases infection during calving or abortion. Thus, herd size and density of animal population together with poor management are directly related to infection rate. Poor management and large herd size contribute to high prevalence of brucellosis [5]. Investigation done by [58] on seroprevalence and risk factors of brucellosis in camels brought for slaughtering at Akaki abattoir, disclosed that age of camels is an important factor affecting the occurrence of brucellosis.
2.9. Treatment:
Brucella organisms are Gram-negative coccobacilli which are sensitive to many broad-spectrum antibiotics [18], but the use of antibiotics is forbidden in many countries because of uncertainty about the infective status and antibiotic resistance. Treatment is unlikely to be cost-efficient or therapeutically effective because of the intracellular sequestration of the organisms, mainly in the lymph nodes [33]. Treatment for human brucellosis includes administration of Tetracycline (five hundred gram every six hours orally) administered for at least six weeks, Doxycycline (a long acting tetracycline analogue) in dose of hundred gram every twelve hours orally with amino glycoside for the first two to three weeks of therapy. Other antibiotic used for treatment are Streptomycin, Gentamicin, Rifampicin, Fluoroquinolones, Trimethoprim or sulfamethoxazole in combination with another agent, such as doxycycline, rifampicin or streptomycin [18].
2.10. Prevention and Control:
The control and prevention of brucellosis depend on animal species involved, Brucella species, management practices and availability and efficacy of vaccines. The options to control the disease include immunization, testing and removal, and improving management practices and movement control[18]. Thus, control by herd immunization and vaccination of calves at four to eight months of age is helpful. Test and slaughter policy can be followed in counties where intensification is practiced [9]. From diagnostic base initial control measures including testing, quarantine and slaughter with vaccination implemented to reduce high prevalence [18]. In Endemic area, treatment can successfully eliminate shedding of organisms from long term carriers, but it is believed to be economically unviable [52]. Effective vaccine against brucellosis in camels and other ruminants is live attenuated B. abortus S19 and B. melitensis Rev-1 proved [33]. Disadvantage of both vaccines are causing abortion, pathogenic to human beings and interference with serological tests. The non-smooth strains of B. abortus RB51 and B. melitensis M111 have recently been introduced into some countries. These vaccines are said to be safe and do not interfere with serological tests [9].
B. abortus “strain 19” or S19 (here after, S19) is an effective vaccine to prevent brucellosis until it was replaced by RB51. Brucella Strain 19 maintains its smooth appearance derived from the presence of the extracellular lipopolysaccharide (LPS). B. abortus strain RB51 vaccine has been developed in United States and tested for its efficacy and safety. This mutant strain of B. abortus does not produce cross-reacting antibodies in vaccinated animal that are detected in the routine surveillance tests. It means that animals vaccinated with RB51 remain negative on the brucellosis surveillance tests and do not give false positive results. This is because Brucella strain RB51 is rough as it lacks the lipopolysaccharide O chain, this feature gives it an advantage because it does not induce the antibodies that are detected by official diagnostic tests, resulting in the differentiation of vaccinated from infected animals [62]. Control of brucellosis in pastoral settings is difficult because of inaccessibility of public and veterinary health services, close contact between animals and their owners, ingestion of unpasteurized dairy products, and seasonal changes in livestock composition. Economic and cultural dependence of pastoral communities on their livestock implementing strategies based on culling infected animals is not acceptable, because animals are primary source of livelihoods. Therefore, the disease has a stable transmission level and tends toward persistence and endemic stability [63].
3. Materials and Methods:
3.1. Study Area:
This study was conducted in Borana zone, which is among the 20 zones found in Oromia National Regional State. The zone has thirteen pastoralist District namely, Arero, Dhas, Dillo, Dirre, Dubluk, Eelwoye, Gomole, Guchi, Miyo, Moyale, Taltale, Yaballo and Wachile, and one town administration Yabello town. Borana zone is located 4˚ 3’ to 5˚ N latitude and 37˚ 4’ E to 38˚ 2’ E longitudes and the landscape is characterized by slightly undulating peaks up to 2000 meters above sea level (masl) in some areas. It shares common boundaries with Guji zone in the east, Somali National Regional State in south east, southern Nation’s Nationalities and Peoples Rational State in the west and one international boundary with Kenya in the south [64]. The area is characterized by bimodal pattern of rain with annual average precipitation ranging from 300mm to 700mm. the main rainy season locally known as ʺGannaʺ extending from mid of March to May and small rainy season termed ʺHagayyaʺ from mid of September to mid-November. The other two seasons are the cool dry season ʺAdoolessaʺ extending from June to August and the major dry season ʺBonaʺ extending from December to February. Animal husbandry in the region is characterized by extensive pastoral productions system and seasonal mobility. Cattle are the dominant animal species followed by goats, camels and sheep [65]. Two districts namely Arero and Elwoye were selected purposively for this study (Figure 3).

Figure 3: Map of Ethiopia and Borena pastoral zone (Developed from Ethiopian shape files using QGIS).
3.2. Study Design:
A cross-sectional study design was conducted from November 2020 to April 2021 by using serological tests, the RBPT and i-ELISA to estimate the prevalence of Brucella infection in camels in the two selected districts. Information on each sampled camel including age, sex, herd size, parity, history of abortion, body condition, herd composition and physiological status of camels were record individually. Interview of pastoralists using questionnaires was conducted to assess the community knowledge and perception on camel brucellosis.
3.3. Sample Size:
The sample size for this study was estimated by the formula given by Thrusfield (2007); N= [1.962 Pexp (1-Pexp)]/d2, Where: n= sample size, Pexp= minimum expected prevalence, 1.96= the value of Z at 95 % confidence interval d= desired accuracy level of 5 %. Therefore, by using the above formula and taking the previous prevalence of 3 %, the minimum sample size at 95 % confidence interval and at 5 % precision or accuracy level, the sample size is calculated to be 45 per district. However, the sample size increased to 315 (increased three times) to increase the precision of the estimates.
3.4. Sampling Method:
The sampling method used in this study was multistage sampling to select peasant associations (PAs), villages (Peasant associations) and herd and then finally the camels. The districts from the zone was selected purposefully based on camel population and abortion history and accessibility the districts to the main road by vehicles. Five pastorals is associations were selected randomly from the two districts selected. From these pastoral associations accessible herds were selected from which 315 were selected conveniently.
3.5. Sample Collection:
3.5.1. Blood collection:
About 8 mL of whole blood was collected from the jugular vein, using plain vacutainer tubes and needles, from each camel aged six months and above. Each sample was labeled using codes specific to the individual animal and herd information. The tubes were tilted on a table overnight at room temperature to allow clotting. Serum was collected by decanting [66]. The serum was stored at -20 0C in Yaballo Regional Veterinary Laboratory. During blood sample collection individual animal history includeing age, sex, herd size, parity; history of abortion, body condition and herd composition and herd size were recorded.
3.6. Laboratory Techniques:
Based on the recommendations of World Organization for Animal Health (OIE) Rose Bengal Plate test (RBPT) and indirect enzyme linked immunosorbent assay (i-ELISA) were used in this study. The i-ELSIA used in this study employ purified LPS antigen with good sensitivity [44].
3.6.1. Rose Bengal plate test:
Equal volume (30 µL) of stained antigen and test serum were mixed and rotated gently up to four minutes on a white tile or enamel plate. Based on the absence and presence of agglutination due to an antigen and antibody complex the result was read as positive or negative. To detect micro-agglutination results of RBPT magnifying glass was used and interpreted as 0, +, ++ and +++. 0 = no agglutination; + = barely visible agglutination; ++ = fine agglutination and +++ = coarse agglutination. Samples with no agglutination (0) were recorded as negative while those with +, ++ and +++ were recorded as positive [12].
3.6.2. Indirect enzyme-linked immunosorbent assay:
The screened samples that positive by RBPT were further confirmed by i-ELISA to detect Brucella antibodies. In this study commercial i-ELISA kit (ID. Vet innovative diagnose ID Screen® Brucellosis Serum indirect Multi-species, BRUS-MSvar 1014GB) used to detect antibodies directed against B. melitensis, B. abortus and B. suis using short incubation method was used. A wash solution was dispensed into each well in 96 well plate pre coated inactivated antigen B. abortus LPS. Specimen and the controls were added into the plate diluted at 1: 20. This mixture was gently shaken, covered with plate sealing tape and incubated at 37 °C for 30 minutes. Each well was washed with the wash solution approximately 300 µL three times to avoid drying of well between washing. The conjugate was added into each well, covered with plate sealing tape and incubated at 37 °C for 30 minutes. The plate with all its wells was re-washed three times with wash solution approximately 300 µL. The substrate was added into each well at room temperature 26 °C for 30 minutes incubated in dark. Finally, 100 µL stop solution was added and the ELISA reader machine was read plate [67].
ELISA reader machine was measure Optical density (OD) at a wavelength of 450 nm. To assess the quality of a plate, the OD was not exceeding 2.00 for positive control and 0.500 for negative control. Results were calculated as percentage of the ratio between the corrected sample OD and positive control OD (S/P-ratio). S was the OD of the test sample minus the OD of the negative control (NCx), over P: the OD of the positive control (PCx) minus the OD of the NCx. S/P %= 100x (Sample –NCx) / (PCx-NCx). A cut-off of ≥ 80 % according to the manufacturer was to be considered for positive test samples [44].
3.7. Questionnaire Survey:Structured questionnaire was used to assess the awareness of the community (both owners and herders) about brucellosis in camels. The structure of the questionnaires focused on the perception and knowledge of the pastoral community about brucellosis in camels and was written in English and translated to local language (Afaan Oromoo). During pre-testing, additional information was gathered and some of the questions were modified. In total, forty-five (45) pastoralists whose animals were test for brucellosis were interviewed. The information gathered by the questionnaire was related the potential routes of transmission in animal and human, clinical signs in animal, species it affects, and measures taken to prevent and control the disease.
3.8. Ethical Clearance:
Written informed consent was obtained from all participants and legal guardians of minors. This study was approved by the Institutional animal care and use committee of Addis Ababa University College of veterinary medicine and agriculture
3.9. Data Management and Analysis:
Data generated from the survey and laboratory investigations were recorded and coded using a Microsoft Excel spread sheet (Microsoft Corporation) and analyzed using STATA version 13.1 for Windows (STATA Corp. College Station, TX, USA). The association between explanatory and outcome variable was analyzed at individual animal level by using logistic regression. Associated risk factors and seroprevalence analysis was conducted with multivariable logistic regression and chi-square test model respectively. Prevalence was compared with the chi-square test as appropriate. Odds ratio was used to assess the strength of association between exposures variables associated with seropositivity of the disease in animals. Foe analysis of the effects of reproductive parameters and seroprevalence the analysis was restricted to female camels. The effects clustering was checked by mixed-effect logistic regression methods. The significance level was set at 5% and 95% confidence level where P value ˂ 0.05 was set statistically significant.
4. Results:
4.1. Seroprevalence of Brucellosis in Camels and Associated Risk Factors:
Of a total of 315 dromedary camels (53 male and 262 female) tested by using RBPT 29 (9.21 %; 95 % CI: 6.25 – 12.95) of them were found positive. When the positive samples were subjected to i-ELISA 9 (2.86 %; 95 % CI: 1.31 – 5.35) of them gave positive results for Brucella infection. The prevalence was higher in camels tested from Elwoye district 4 (3.17 %; 95 % CI: 0.87 – 7.93) than those tested from Arero district 5 (2.65 %; 95 % CI: 0.86 – 6.06). The results serological test was given in Table 4.
Camels which were tested from large herd size were more likely to test positive for anti-Brucella antibodies those which were tested from small herd size (OR = 17.04; 95 % CI: 1.77 - 164.04) (Table 5). Female camels with the history of abortion had higher prevalence brucellosis than those without history abortion, which was statistically significant difference (OR = 6.24; 95 % CI: 1.08 -35.86) (Table 6).
|
Variables |
No. examined |
No. Positive
|
Prevalence |
X2 |
P-value |
||||
|
District |
|||||||||
|
|
Arero |
189 |
5 |
2.65% |
0.08 |
0.783 |
|||
|
Elwoye |
126 |
4 |
3.17% |
||||||
|
Sex |
|||||||||
|
|
Female |
262 |
8 |
3.05% |
0.24 |
0.645 |
|||
|
Male |
53 |
1 |
1.89 % |
||||||
|
Age |
|||||||||
|
|
Young |
83 |
1 |
1.20 % |
1.87 |
0.1716 |
|||
|
Adult |
53 |
1 |
1.89% |
||||||
|
Old |
179 |
7 |
3.91% |
||||||
|
Herd size |
|||||||||
|
|
Small |
106 |
1 |
0.94% |
9.02 |
0.0027 |
|||
|
Medium |
167 |
3 |
1.8% |
||||||
|
Large |
42 |
5 |
11.9% |
||||||
|
Parity |
|||||||||
|
|
No parity |
87 |
1 |
1.15% |
1.87 |
0.1710 |
|||
|
Single parity |
62 |
2 |
3.23% |
||||||
|
Two and more |
113 |
5 |
4.42% |
||||||
|
Reproductive problem history |
|
||||||||
|
|
Abortion |
40 |
4 |
10 % |
5.51 |
0.014 |
|||
|
RFM |
36 |
2 |
5.56% |
0.74 |
0.358 |
||||
|
Stillbirth |
30 |
1 |
3.33% |
0.01 |
0.925 |
||||
|
Body condition |
|||||||||
|
|
Poor |
148 |
5 |
3.38% |
0.22 |
0.6380 |
|||
|
Medium |
84 |
2 |
2.38% |
||||||
|
Good |
83 |
2 |
2.41% |
||||||
|
Herd composition |
|||||||||
|
|
Camel only |
48 |
1 |
2.08% |
0.68 |
0.4101 |
|||
|
Camel &Bovine |
49 |
1 |
2.04% |
||||||
|
Camel & shoats |
92 |
2 |
2.17% |
||||||
|
All specious |
126 |
5 |
3.39% |
||||||
Table Table 4: Results of Univariable analysis to identify risk factors
|
Risk factor |
Odds ratio |
Std. Err. |
Z |
P>z |
[95%Conf. |
Interval] |
|
District |
|
|
|
|
|
|
|
Elwoye |
1.160226 |
0.9025108 |
0.19 |
0.848 |
0.252589 |
5.32931 |
|
Sex |
|
|
|
|
|
|
|
Female |
0.6221431 |
0.8071894 |
-0.37 |
0.715 |
0.048923 |
7.911663 |
|
Age |
|
|
|
|
|
|
|
Adult |
1.927058 |
2.832757 |
0.45 |
0.655 |
0.108053 |
34.36789 |
|
Old |
5.588583 |
7.002644 |
1.37 |
0.170 |
0.4794279 |
65.14484 |
|
Body condition |
|
|
|
|
|
|
|
Medium |
0.7789702 |
0.6905897 |
-0.28 |
0.778 |
0.137055 |
4.427379 |
|
Good |
0.9508267 |
0.8709119 |
-0.06 |
0.956 |
0.1579215 |
5.724814 |
|
Herd size |
|
|
|
|
|
|
|
Medium |
1.829599 |
2.194525 |
0.50 |
0.615 |
0.1743316 |
19.20152 |
|
Large |
17.03541 |
19.68525 |
2.45 |
0.014 |
1.769079 |
164.043 |
|
Herd composition |
|
|
|
|
|
|
|
Camel & Bovine |
0.9690467 |
1.460472 |
-0.02 |
0.983 |
0.0505219 |
18.58703 |
|
Camel & Shoats |
1.346338 |
1.753916 |
0.23 |
0.819 |
0.1047774 |
17.29979 |
|
Camel, Shoats & Bovine |
1.572648 |
1.928595 |
0.37 |
0.712 |
0.1421582 |
17.39766 |
Table 5: Results of multivariable analysis to identify risk factors
|
Risk factor |
Odds ratio |
Std. Err. |
Z |
P>z |
[95% conf. |
Interval] |
|
Parity |
|
|
|
|
|
|
|
Single parity |
1.788536 |
2.331348 |
0.45 |
0.656 |
.1389827 |
23.01625 |
|
More than one |
2.295611 |
2.770359 |
0.69 |
0.69 |
.2156067 |
24.44186 |
|
Abortion |
|
|
|
|
|
|
|
|
6.23754 |
5.566409 |
2.05 |
0.040 |
1.084919 |
35.86158 |
|
Stillbirth |
|
|
|
|
|
|
|
|
.6958434 |
.7892473 |
-0.32 |
0.749 |
.0753446 |
6.426445 |
|
RFM |
|
|
|
|
|
|
|
|
.5955831 |
.5905866 |
-0.52 |
0.601 |
.0852868 |
4.159134 |
|
_cons |
.0117072 |
.0117072 |
-4.42 |
0.000 |
.0016303 |
.084068 |
Table 6: Result of association between seroprevalence and reproductive parameters.
4.2. Results of Questionnaire Survey:
4.2.1. Sociodemographic characteristics of respondents:
A total of 45 respondents interviewed during this study which 27 (60%) of them were from Arero and 18 (40%) were from Elwoye districts. The majority 36 (80 %) of the participants was males and the remaining 9 (20 %) were females. When their age is considered 53.33% participants were between 25 to 45 ages. Majority of the participants were camel owner 29 (64.44%) while other is camel herder 16 (35.56%). Most of the animal herders had greater knowledge about the brucellosis than camel owner which was statistically significant (P<0.05) (Table 7).
|
|
||||
The level of respondents’ knowledge regarding brucellosis was high; 33 (73.33 %) respondents knew about the disease which is locally known as “salleessa/salleessisa” (figure 4). Most of them had heard about brucellosis from their family, neighbors and Personal observation 31 (93.94 %) whereas others got information from traditional healers 4 (12.12 %) and animal health workers 1 (3.03 %).
The pastoral communities have been living with their animals for generations and have built enormous indigenous knowledge on animal health problem. Knowledge transfer from the animal health worker to the society is a key intervention for the prevention, control and eradication of disease. The eminent gap of perception of the society about animal disease was due to the absence of well-designed attempt of animal health extension service, Poor infrastructure that constrain access to mobile livestock communities, integration of the CAHWs system into the veterinary service and limited resources to maintain service delivery.
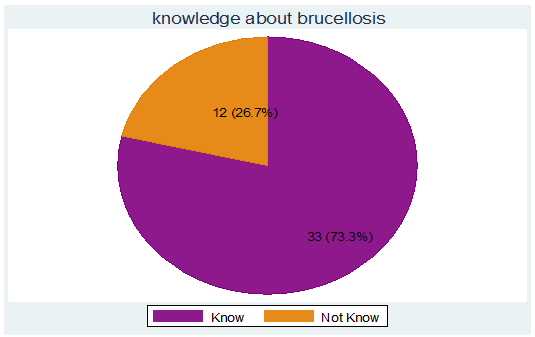
Figure 4: Respondents’ knowledge about brucellosis
The participants disclosed that they have been aware about the symptoms of brucellosis in camels. Although few differences was observed in signs described by the pastoralists, the majority revealed that common sings of brucellosis include abortion, RFM and stillbirth with 100%, 81.82% and 66.67%. There was similar knowledge between camel owners and herders with regard to recognition of signs of brucellosis in camels (figure 5).
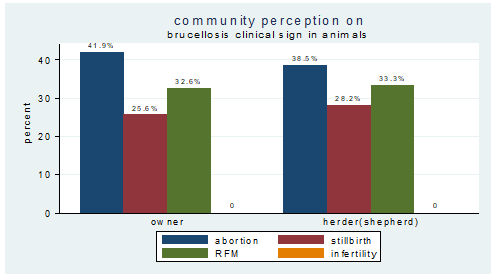
Figure 5: Respondents’ knowledge about sign of brucellosis in camel between owner and herder
|
Variable |
Category |
Percent % |
Frequency(N=45) |
|
Do you know brucellosis |
Yes |
73.33% |
33 |
|
No |
26.67% |
12 |
|
|
Your source of information |
Health care workers/ CAHWs |
3.03% |
1 |
|
Neighbors/family/Personal observation |
93.94% |
31 |
|
|
Traditional Healer |
12.12% |
4 |
|
|
FM-Radio |
_ |
_ |
|
|
Level of Knowledge from who claimed knowledge of brucellosis |
|||
|
Variable |
Category |
Percent % |
Frequency(N=33) |
|
Animal specious it affect |
Camel |
100% |
33 |
|
Cattle |
100% |
33 |
|
|
Goat |
100% |
33 |
|
|
Sheep |
21.21% |
7 |
|
|
|
Wild animals |
_ |
_ |
|
Symptoms in animals |
Abortion |
100% |
33 |
|
Retain placenta |
81.82% |
27 |
|
|
Stillbirth |
66.67% |
22 |
|
|
Swollen leg joints |
_ |
_ |
|
|
Infertility |
_ |
_ |
|
|
reduced milk production |
_ |
_ |
|
|
Do you know brucellosis as zoonotic disease |
Yes |
18.18% |
6 |
|
No |
81/82% |
27 |
|
|
Symptoms in man |
Fever |
_ |
_ |
|
Joint pain |
3% |
1 |
|
|
Headache |
_ |
_ |
|
The majority 27 (81.82 %) of the participants were not aware about the transmission methods of brucellosis between domestic animals and humans. Concerning the zoonotic nature of brucellosis only 6 (18.18 %) of respondents knew that it is transmitted from animals to humans, of which 5 (15.15 %) mentioned consumption of raw milk as most common mode of transmission from. Transmission from animals to animals was mentioned by 9 (27.3%) of the pastoralists to by mixing of different animals species and contact with aborted materials was indicated by 2 (6.06 %) of the respondents. Different and common overall seasonally occourance of brucellosis in animals mentioned by respondents are Major Rainy Season (“Ganna”), Cool Dry Season (“Adololessa”), Short Rainy Season (“Haggaya”) and Major Dry Season (“Bona”) with 93.94%, 24.24%, 21.21% and 9.09%. The details of the results of questionnaire are presented in (Table 9).
|
|
Variable |
Category |
Percent(%) |
Frequencies(N33) |
|
|
|
Source of infections for animals |
contact with aborted material |
6.06% |
2 |
|
|
Sharing the same pasture/water |
_ |
|
|||
|
|
|
|
|
||
|
Introducing brucellosis infected animal into a herd |
_ |
_ |
|||
|
Mixing with brucellosis infected or different domestic animals |
27.3% |
9 |
|||
|
|
Sex more affected |
Male |
_ |
_ |
|
|
Female |
100% |
33 |
|||
|
|
Age Brucellosis more common in animal |
Young (< 3 years ) |
_ |
_ |
|
|
Adult (3 - 4 years) |
_ |
_ |
|||
|
Old ( > 4 years) |
100% |
33 |
|||
|
|
Season Brucellosis more prevalent |
Major Rainy Season (“Ganna”) |
93.94% |
31 |
|
|
Cool Dry Season (“Adololessa”) |
24.24% |
8 |
|||
|
Short Rainy Season (“Haggaya”) |
21.21% |
7 |
|||
|
Major Dry Season (“Bona”) |
9.09% |
3 |
|||
|
Transmission methods to man |
Consuming raw milk |
15.15% |
5 |
|
|
|
Consuming raw meat/ blood |
_ |
_ |
|
||
|
Contact with aborted fetus |
_ |
_ |
|
||
|
No idea |
84.85% |
28 |
|
||
All Respondents 100% described that aborted material and other excreta are handled with bare hands, and they did not use any protective material while handling parturient livestock, removing placenta and other aborted materials. With limited knowledge about their responsibilities in the prevention and control of zoonotic disease, animal and human health care workers are not equipped to advise the public on appropriate prevention and control strategies. Some of this lack of knowledge can be explained by structural or institution
|
Variable |
Category |
Percent (%) |
Frequency(N=33) |
|
Use personal protective when Delivery assistance or contact aborted material |
Yes |
_ |
_ |
|
No |
100% |
33 |
|
|
Proper disposing aborted foetus/fetal membrane |
Yes |
_ |
_ |
|
No |
100% |
33 |
|
|
Mating assistance |
Yes |
100% |
33 |
|
No |
_ |
_ |
|
|
Intervention you take if animals have brucellosis |
Isolate |
_ |
_ |
|
Cull |
6.06% |
2 |
|
|
Self-treatment |
15.15% |
5 |
|
|
Take to clinic |
_ |
_ |
|
|
Do nothing |
78.79% |
26 |
|
|
Raw milk consumption |
Yes |
100% |
33 |
|
No |
_ |
_ |
|
|
Raw meat consumption |
Yes |
100% |
33 |
|
No |
_ |
_ |
|
|
Milk usage |
For Sale |
100% |
33 |
|
For family |
100% |
33 |
Table 10: Community practice regarding brucellosis in study area
Camel brucellosis sero-positive in herd level was insignificant (p > 0.05) different association between respondents those who know and not know brucellosis (Table 11).
|
Respondents knowledge level |
OR |
Std. Err. |
Z |
P>|z| |
[95% Conf. Interval ] |
|
|
Know Brucellosis |
Yes |
- |
- |
- |
- |
- |
|
No |
3.625 |
2.94 |
1.59 |
0.113 |
0.74 - 17.81 |
|
|
Constant. |
0.137 |
0.07 |
-3.71 |
0.000 |
0.05 - 0.39 |
|
Table 11: Univariable logistic regression analysis of respondent level of knowledge regarding Brucellosis for Brucella seropositivity found at herd level
Camel production has been considered an important economic activity in Borana pastoral area and remains so in the future. However, the optimal utilization of this important resource can be impaired by infectious diseases such as brucellosis. Brucellosis affects the productivity and reproductive efficiency of animals through reduction of milk production, abortion and decreased fertility [68]. This study provides important information on the occurrence of brucellosis in camels in Borana pastoral zone. Although the prevalence observed is low, it is not without impacts. Since animals and humans live intimately in the area sometimes sharing shelters the occurrence of brucellosis in camels has important implication for public health. Thus, it is an addition to the existing information on brucellosis in livestock. Previously the occurrence of brucellosis has documented in other livestock species [61]. The prevalence of brucellosis observed in this study in camels is in close agreement with the 2.43 % prevalence reported by [69] in Jijiga and Babile, eastern Ethiopia; the 2.09 % prevalence reported by [5] in Afar, Northeastern Ethiopia; the reports of [42] and [70] who observed a 3 % prevalence in southern Ethiopia and that of [12] and [70] who reported similar prevalence in camels in Tigray, Northern Ethiopia. However, the results of this study are higher than that findings of [57], [59], [71], [4], [72] and that of [11] who reported lower sero-prevalence from different parts of the country. On the other hand the results this study is lower than some of the reports done elsewhere in the world. For instance, it is lower than the prevalence of 5.8 % reported from Sudan [9], 5.7% from Libya [25], 10.5% recorded in Nigeria [30], 11.5% in Egypt [66], 14% and 15.36% in Kenya [73] and [26], respectively, 8.15% in Iran [16], and 9.09% in, Pakistan [24].
The difference observed could be due differences herding structure, the laboratory tests used and the sample size used for the investigation. In the current study area camels and other livestock species owned by different individuals and communities are often herded together. In contrast in places where most of the previous studies were under taken there is clan-based herd segregation, which is likely to reduce introduction and spread of Brucella among herds or animals. The sensitivity and specificity of the confirmatory test also vary and may have contributed to the variation in prevalence. The differences could also be due to variations in animal management practices, the number of susceptible camels, presence of high number of camels in the herds and mixing of aborting camels with others and absence of accessible preventive veterinary services, close contact with infected domestic and wild animals, population intensity, lack of awareness about the disease in camels [68]. In relation to husbandry practices, these animals are usually kept overcrowded and reared in open system without differentiation of aborted and pregnant ones and housed together with high stocking density, all these factors play important role in the spread of the infection. The lack knowledge on the mechanisms of transmission of Brucella species might have caused Brucella infected animals used for breeding purpose which serves as source of infection [74].
Herd size was highly statistically significant (P=0.017) risk factor for camels brucellosis in this study. It is likely that the risk of disease transmission increased in a large herd size this is in accordance with the findings of [32] in the Afar region of Northeast Ethiopia, [72] in selected districts of afar region, Ethiopia and [5] in Selected Pastoral Districts of Afar, Northeastern Ethiopia. As herd size increases, the chance of contact between animals increases leading to more chances of infection particularly during calving and abortion [54]. As herd size increases, the chances of contact between animal also increases, leading to more chances of infection which is particularly more important during calving or abortion when maximum brucellosis contamination occurs. Thus, herd size and density of animal population together with poor management are directly related to infection rate [5]. Owning large herd considered as all the animals in the herd were productive. Therefore, low milk yield and infertility of some animals was not considered as a threat to the overall productivity which allowing them to keep chronically sick animals and less productive. These sick animals have other values attributed to them, such as infertile animals being valued for their size or animal with low milk yield being thought of as calmer. Consequently, as a result of these attitudes the spread of brucellosis in the herd is high [75]. Lower prevalence in Small herd size could be associated with grazing at the pasture near to enclosure without long distance movement, easy to manage and identify sick animal which minimize predisposing factors and avoid contact with other herd.
Abortion, retained fetal membrane and stillbirth were reproductive problems obtained in the history of the adult female camels with prevalence of, 10 %, 5.56% and 3.33% respectively. Statistically significant (p < 0.05) difference sero prevalence was observed in abortions which close agreement with [54] in Selected Districts of Afar, Ethiopia, [12] Mehoni District, southeastern Tigray, Ethiopia, [70] in Yabello district of Borena Zone, southern Ethiopia, [59] in and Around Dire Dawa City , eastern Ethiopia, [11] in Fentale district Oromia Regional State, Ethiopia, [69] in Jijiga and Babile districts, Eastern Ethiopia, [14] in the Sultanate of Oman and [20] in Yemen. Camel herd kept in close contact with bovine and small ruminants in the current study sero prevalence were 2.08% in camel kept alone, 2.04% camel kept with bovine, 2.17%camel kept with small ruminants and 3.39% camel kept with bovine and small ruminants. However, no statistically significant difference was observed between these four camel groups. The present finding was in line with the observation in Yabello District of Borena Zone, Southern Ethiopia by [70] and in Southern lowland of Ethiopia by [42]. The results of my study go parallel with the findings of [5] in Afar.
Animal species diversification is common in Ethiopia and has economic and ecological advantages. However, it increases the chance of brucellosis and other disease transmission from other infected ruminants to dromedaries [32]. spread of the disease among animal may be mixing of different specious during movement for grazing and watering in the dry season as aggregating the animals around watering point might increase the contact between infected and healthy animals and thereby facilitate the spread of the disease [42]. Body condition of the camels was considered in this study to see the distribution of the infection in different body condition. Even though, in this study body condition score was statistically not significant (P>0.05), high seropositivity was found in camels with poor 3.38% and medium 2.38% body condition than camels with good 2.41% body condition. Body condition of the camels was considered in underfed animals are expected to have a poor body condition that is manifested by decreased immunity against various infections [54]. Nutrition plays a great role in Immunity against various infectious diseases. Underfed animals are expected to have a decreased immunity that is manifested by poor body condition [58].
Age was classified as young (< 3 years), adult (3-4years) and old (>4) based on sexual maturity to see the distribution of diseases in each age group. Accordingly, this study reveals that brucellosis infection may occur in animals of all age groups, but commonly persists in old camels (3.91%) than in adult (1.89%) and young (1.20 %). Although no statistically significant difference (P>0.05) was observed between each age groups, being higher in the sexually mature age group. Susceptibility to brucellosis in camels of different age groups slightly higher in older animals which is in agreement with the previous reports of [59], [71], [32], [76], [54], [69], [4], [14] and [78] [44]is infection may occur in animals of all age groups but persists commonly in older and adult animals which sexually mature due to presence of growth factors (erythritol and hormones) which favors the multiplication of pathogen in sexually mature animals [58]. Animals which older and adult are at risk of infection due to sexual mating and diseases transmission [54].
Brucellosis in all age groups of camels indicates that infection started early in life probably through sucking and persisted into adulthood [78]. Younger animals tend to be more resistant to infection due to less development sex hormones and erythritol which stimulate the growth and multiplication of Brucella organisms, tend to increase in concentration with age and sexual maturity [78]. Although a few latent infections, frequently clear infections [4] and less exposure may occur. Sero-prevalence of camel brucellosis according to sex was higher in female 8 (3.05 %) than male 1(1.89 %), This is consistent with previous study of [11], [4], [42] [58], [59], [71], [77], [72], [5], [70], Warsame et al., (2012) from Ethiopia, [9] from Sudan, [79], [24] from Sindh Pakistan and [19] in the south province of Jordan. Although no statistically significant difference (P>0.05) was observed between sex.
Females are at high risk of brucellosis than males due to their usefulness in the production herds, females generally have a longer lifespan than males, and this may have increased exposure to the bacterium [14]. Reduction of immunity in females during lactation, pregnancy and other reproductive stress may also contribute to higher prevalence in female camels [66]. There was no statistically significant difference between the sex groups in the current study. These results may be associated with the effect of erythritol in both sex [66]. The number of breeding males kept by the pastoralists in the camel herds is very small on which random sampling method was applied and this predictably bias the statistical analysis [32]. On the contrary [70], [4], [12] and [69] in Ethiopia and [80] in Sudan and [78] in Mongolia reported the occurrence of infection is higher in male than female animals. This might be due to the number of breeding males kept by the pastoralists in the camel herds of the present study was very small on which random sampling method was applied and [70].
Parity was statistically insignificant difference (P>0.05) in this study. Prevalence of she-camels with the history of no parturition, Primiparous and Pluriparous were 1.15%, 3.23% and 4.42%, respectively. Therefore, this is consistent with the previous study by [32] and [54] in Afar, [70] in Yabello District of Borena Zone, Southern Ethiopia, [42] in Southern lowland of Ethiopia and [43] in selected districts of Punjab, Pakistan. This might be due to repeated exposure of the she- camels to parturition and other physiological stress increases the probability of acquiring Brucella infection [70]. On the contrary [59] reported the occurrence of infection is higher in single parturition than no parturition two and more female animals [54]. Identifying the level of community perception regarding brucellosis contributes to control at the human-animal interface through awareness creation. Understanding of participants toward brucellosis in animal was seen in a large proportion of (73%) participants. This is in line with previous reports in Ethiopia [81], Uganda [82] and Kenya [83] but differed from others studies conducted in Egypt [84], Malawi [85], Pakistan [86] and [87]. Pastoralist communities have been living with their animals for generations and have built enormous indigenous knowledge with animal health problem but concerning brucellosis being zoonosis Only 6 (18.18%) participants could correctly identify that diseases can be transmitted from livestock to humans.
The accessibility of information related to brucellosis, the main sources of information in study area were from family, neighbor and personal observation 31 (93.94 %). About 4 (12.12 %) and 1 (3.03 %) of participants said that they have heard about brucellosis from traditional healer and health care workers or CAHWs respectively. FM-Radio were not a source the information for pastoralist community in study area. This in line to Surveys conducted in Uganda [82]. Contrary to this finding the study in Kenya [83] found CAHWs were the main source of information for pastoralist. Knowledge transfer from the animal health care worker or CAHWs to the society is a key intervention for the prevention and control of disease. The eminent gap of perception of the society about animal disease from animal health worker was due to the absence of well-designed attempt of animal health extension service, poor infrastructure that constrain access to mobile livestock communities, absence of integration system of CAHWs into the veterinary service and limited resources to maintain service delivery [88].
Almost all participants were knowledgeable about the brucellosis susceptibility of different animal species because of rearing different livestock specious for diversification which has economic and ecological advantages and increase community awareness regarding disease in livestock. Identifying brucellosis affected species and its symptoms are crucial for livestock owners’ practices towards prevention and control measures of brucellosis. In this study all participants who claimed to know brucellosis had high level of knowledge of animal brucellosis clinical signs, mostly recurrent abortion, RFM and stillbirth in contrasts with studies in Kenya [83], conversely very low knowledge of the symptoms of brucellosis was fund infertility, reduced milk production and swollen leg joints which similar to studies done in Uganda [82] and Egypt [89]. The major risk factors of brucellosis transmission among different animal specious was mentioned by 9 (27.3%) respondents as mixing animal with brucellosis infected different livestock and through contact with aborted material was identified by 2 (6.06%) of the participants. With regard to public health importance of brucellosis majority 27 (81.82 %) of the participants were not sure that brucellosis can be transmitted from animals to humans. Only 5(15.15%) participants could identify that brucellosis can be transmitted from livestock to humans via raw milk consumption. It comparable with earlier findings in Ethiopia [90] and [91], Pakistan [86], Kenya [83] and Uganda [82]. In pastoral community raw milk consumption was regularly used as a replacement for drinking water however, consumption of raw animal products and close contact with animals were not perceived as risk factors for a disease. This relates to a long-standing traditional practice engaging in increase transmission of disease. Low awareness about brucellosis transmission by eating habits or animal management practices makes communities vulnerable to disease, threatens livestock and cause economic losses [92].
Knowledge gap that lead community to engage in high risk practice such as improper disposal of aborted materials on field and assist their animals in the parturition without any protective wearing identified as priority problem in study area. These findings were in the same line with [81] and [93] in Ethiopia. Combined factors of handling abort material without protective gloves and poor cleaning practice could pose a great risk of disease spread to human and animals. Aborted fetuses and the placenta Proper handling decrease incidence and environmental transmission of brucellosis. According to our study, 2 (6.06%) participants may sell frequently aborted animals in their herds, potentially increasing brucellosis transmission not only between households in the same village, but also across larger geographical areas. This finding is similar to study in Ethiopia [93], Jordan [94] and Egypt [95]. Participant mentioned actions taken when confronted with aborting animal in the herd ware without seeking veterinary services give treatment by themselves and majority of them would do nothing without isolate brucella infected animal. Failure to isolate suspected animals has been one of the major risk factors for transmission of Brucellosis within and between herds [83].
Only a small proportion of respondents perceived that brucellosis was a serious disease in animals and humans but they had unfavorable attitude towards prevention of brucellosis. Lacks of awareness of brucellosis were more likely make them to engage in risky practices that could expose them to infection. Effective control strategies cannot be currently implemented due to the lack of awareness high-risk practices like close contact between animals, ingestion of unpasteurized dairy products, and different livestock composition. Economic and cultural dependence of pastoral communities on their livestock implementing strategies based on culling infected animals is not acceptable [63]. Because of a lack of community awareness high-risk activities and lack of effective prevention and control measures are currently unavailable. Control programs could be more successful by educating livestock owners/herder and change their behavior towards disease control and animal health. Knowledge, attitude and practices survey play a vital role in evaluating livestock owners’ understanding and preparedness against such livestock diseases [87].
5.1 Study limitations:
As most of the survey was conducted in drought season, some of pastoralists refused to allow collecting blood sample while animals are in poor body conditions. Seasonal migration of livestock in Borana in search of good pasture and watering points could be associated with temporal variation of prevalence of the disease that was not assessed due to the cross-sectional design of the current study. The major limitation of the study was the small sample size of questionnaire which could affect the power of the study and external validity of the findings making it impossible to generalize findings even to the whole.
5.2 Acknowledgements:
Above all, all praises and thanks are for Almighty ALLAH, Most Merciful and Most Gracious, who’s enabled me to perceive and pursue ideas of life, blessed me with good health and proper guidance. My special thanks also to my Father Giro Dika and my mother Loko Jarso, who has been giving me unpaid sacrifice throughout my life.
6. Conclusion and Recommendations:
The results of present study revealed that of the prevalence of camel brucellosis in Borana zone was low but it is enough to affect animal health, human health and the economy. Among the potential risk factors assessed only large herd size and history abortion were significantly associated with camel brucellosis in the area. The majority of the community had moderate overall knowledge regarding camel brucellosis but most of the community had no knowledge about the zoonotic importance of brucellosis, its transmission mechanisms, consequences of consuming raw milk, handling aborted fetus and fetal membranes without any protective materials. Therefore, the veterinary and public health authorities as well as the extension service should take the results of this study into account when planning livestock and public health improvement programs.
Therefore, based on the above conclusion the following recommendations were forwarded: