Agricultural Research Pesticides and Biofertilizers
OPEN ACCESS | Volume 6 - Issue 1 - 2026
ISSN No: 2994-0109 | Journal DOI: 10.61148/2994-0109/ARPB
Miki Tanaka1, Yuzuri Iwamoto1,5*, Tomoaki Kouya2, Kenta Moriyama1, Kazuya Tomimoto1, Yasuyuki Shitomi1, Tohru Hayakawa1,6, Delwar M. Hossain1,7, Shin-ichiro Asano3, Masayuki Taniguchi2, Masaaki Azuma4, Hidetaka Hori1,8*
1Laboratory of Applied Biochemistry, Graduate School of Science and Technology, Niigata University, Niigata, Japan.
2Laboratory of Chemistry and Chemical Engineering, Graduate School of Science and Technology, Niigata University, Niigata, Japan.
3Graduate School of Agriculture, Hokkaido University, Sapporo, Hokkaido, Japan.
4 Faculty of Agriculture, Tottori University, Tottori, Japan.
5Current Address: Department of Applied Chemistry and Bioscience, Graduate School of Engineering,
Kanagawa Institute of Technology, Atsugi, Kanagawa, Japan.
6Current Address: Department of Applied Chemistry and Biotechnology, Okayama University, Okayama, Japan.
7Current Address: Department of Plant Pathology, Bangladesh Agricultural University, Mymensingh, Bangladesh.
8Current Address: AAG Corporation, Daigiri, Fujisawa, Kanagawa, Japan.
*Corresponding authors: Yuzuri Iwamoto, Department of Applied Chemistry and Bioscience, Graduate School of Engineering, Kanagawa Institute of Technology, Atsugi, Kanagawa, Japan.
Hidetaka Hori, AAG Corporation, Daigiri, Fujisawa, Kanagawa, Japan.
Received: November 18, 2021
Accepted: December 01, 2021
Published: December 13, 2021
Citation: Miki Tanaka, Yuzuri Iwamoto, Tomoaki Kouya, Kenta Moriyama, Kazuya Tomimoto, Yasuyuki Shitomi, Tohru Hayakawa, Delwar M. Hossain, Shin-ichiro Asano, Masayuki Taniguchi, Masaaki Azuma, Hidetaka Hori. (2021) “Bacillus thuringiensis Cry2Aa toxins bind to v-ATP synthase subunit A located on the midgut brush border membrane of Bombyx mori.” Journal of Agricultural Research Pesticides and Biofertilizers, 2(5); DOI: http;//doi.org/11.2021/1.1049.
Copyright: © 2021 Yuzuri Iwamoto and Hidetaka Hori. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Bacillus thuringiensis Cry2Aa toxin shows activity against Lepidoptera and Diptera; however, the mechanism responsible for this dual toxicity has not yet been fully elucidated. To further clarify the entomocidal mechanism, we examined the binding between Cry2A toxin and brush border membrane (BBM) prepared from the silkworm Bombyx mori. We previously bio-assayed the activity of the Cry2Aa crystal, showing that Cry2Aa 60 kDa protoxin (Cry2Aa-60kD) and truncated 50 kDa toxin (-50kD) had insecticidal activity against B. mori and Lymantria dispar. In the current study, we labeled purified Cry2Aa-60kD and -50kD with the fluorescent dye Cy3, and the binding between the labeled toxins and BBM of B. mori was quantitated by a Scatchard plot, demonstrating binding coefficients (Kd values) of 97 and 38 nM, respectively. Ligand blot analysis using antiserum raised against Cry2Aa protoxin demonstrated that the solubilized 68 kDa and 60 kDa BBM proteins bound to both the Cry2A-60kD and -50kD. Although the purified active Cry1Aa toxin did not bind to these BBM proteins, it clearly bound to several BBM proteins of 250-110 kDa in SDS-PAGE. By contrast, Cry2Aa did not bind to any of these proteins. Nine internal peptides of the 68 kDa and seven peptides of the 60 kDa BBM proteins showed homology to vacuolar-type ATP synthase subunit A with matrix-assisted laser/desorption ionization time-of-flight mass spectrometry. Five of these peptides overlapped, suggesting a total of 11 peptides with different amino acid sequences exhibiting homology to ATP synthase subunit A. One peptide obtained from the 60 kDa BBM protein matched the internal peptide of the glycosyl hydrolase family 31 protein. These findings help to understand the binding and modification of toxins in the insect midgut which can provide the information to overcome emerging B. thuringiensis toxin resistance.
1. Introduction:
Bacillus thuringiensis (Bt) produces insecticidal crystal proteins that are solubilized with alkaline insect midgut fluid upon ingestion by the insects, resulting in soluble protoxins with a molecular weight of either 130 kDa or 70 kDa [1]. These protoxins are further processed to their respective active toxins [1, 2] via proteinaceous reactions. The active toxin of Cry1A binds to candidate receptor proteins such as cadherin-like protein (CadLP) [3-5] and aminopeptidase N (APN) [6, 7], along with other proteins such as alkaline phosphatase (ALP) [8] and red fluorescent proteins, including chlorophyll-binding proteins (e.g., P252) [9, 10]. Recently the ATP-binding cassette complex (ABCC) was suggested to be a receptor for Cry1A and Cry2 toxins [11-14].
Bt toxin in spray form and in genetically modified crops is considered to be an environmentally friendly insecticide; however, the efficacy of this method is threatened by emergent Cry toxin-resistant insects. Cry1A toxin resistance is currently the most serious issue, as this Bt toxin is one of the most commonly applied and Bt toxin-resistant strains of Plutella xylostella, Helicoverpa armigera, and Trichoplusia ni have been reported [15, 16]. Tabashnik et al. [17] proposed that the emergence of Cry1A toxin-resistant insects could be avoided by alternately using Cry1A and Cry2A toxins. This method was employed to the genetically modified cotton variety Bollgard II that expresses both the Cry2Ab and Cry1Ac toxins. In addition, Bt rice varieties that produce Cry1Ac, Cry2Aa, and Cry1Ca toxins are effectively resistant against pest insects, with no reported effects on non-target species, including spiders and the brown plant hopper [18]. However, to continue producing effective Bt plants, elucidation of the mechanism by which Cry2A toxin kills pests and resolution of the various discrepancies found in the current literature are necessary. Despite great advances in understanding Cry2A toxins, further research is needed to fully elucidate how Cry2A toxin is processed in the midgut of susceptible insects and the killing mechanism of the toxin. Moreover, the receptor for the toxin has not been identified to date.
The insecticidal Cry2Aa toxin was first identified in Bt kurstaki HD-1 [19], with several characteristics that make it distinct from Cry1A toxin. Indeed, the homology between Cry2Aa and Cry1Aa toxins is only 20% at the amino acid level even though their three-dimensional structures are highly similar [20, 21]. Cry2Aa toxin shows dual entomocidal activity against lepidopteran and dipteran insects. A heterologous competition assay showed no competition between Cry2A and Cry1A toxins, which was attributed to their different binding sites in the brush border membrane (BBM) [22-24].
Cry4Ba toxin binds to ATPase subunit A or B in the larvae of the mosquito [25]. Similarly, Cry1Ac toxin was shown to bind to ATPase in Heliothis virescens [26] and H. armigera [27]. Qiu et al. [28] also reported that vacuolar (v)-type ATPase subunit A and B bound to Cry2Aa toxin in Spodoptera exigua, along with several other proteins such as polycalin, 4-hydroxybutylate CoA transferase, and protein kinase C, based on in vitro assays. However, in gene knockdown assays utilizing RNA interference, only interference of v-ATPase subunit B significantly decreased the susceptibility of S. exigua to Cry2Aa toxin [28]. ALP, APN, and CadLP, which are known to bind Cry1A toxins, were also reported to bind Cry2Aa toxin in H. armigera, although ATPase did not bind to Cry2Aa toxin [29].
On the other hand, in the research for development of new type of biopesticides, synthesized double stranded RNA (dsRNA) encoding v-ATPase subunits was applied and significant effects to protect damage from the various pest insects was reported. We briefly list up these interesting trials in the discussion. To elucidate the insecticidal mechanism of Cry toxins, various phenomena regarding activity of Cry toxins should be described in a wide variety of insects belonging to different families. Because parallel evolution is often observed among insects in which the insects distantly related evolve similar features, therefore, a phylogenetic tree is often not useful for understanding the mechanisms of resistance even in lepidopteran insects. This necessitates collecting data from various species and contexts to identify all possible binding modes between the BBM proteins and Cry toxins using various comparative biochemical entomological methods. In other words, prudent judgment is needed in comparing the various phenomena of Cry toxins in different insects for appropriate interpretation and generalization of the underlying mechanisms. Such comprehensive and systematic research approaches using a wide variety insect can help to resolve the various current discrepancies in understanding the mechanisms of Cry toxin receptors.
In previous study, we reported that the Cry2Aa 50 kDa toxin (Cry2Aa-50kD) killed both Bombyx mori and Lymantria dispar [30]. In the present study, we further explored the binding between Cry2Aa toxin and BBM vesicle (BBMV) of B. mori. Through in vitro assays with purified toxins, along with Western blotting assay using the antisera, we found that the Cry2Aa toxin binds to 60- and 68-kDa solubilized BBM proteins having homology to v-ATP synthase subunit B. Moreover, although Cry1Aa toxin could also bind to various BBM proteins with molecular sizes of around 250–110 kDa, neither Cry2Aa toxin bound to these BBM proteins. These results are expected to help clarify the toxic mechanism of Cry2Aa toxin in the future.
2. Materials and Methods:
Insect Rearing:
The hybrid B. mori strain Shunrei × Shogetsu was reared on an artificial diet as described by Asano et al. [31]. One-day-old fifth-instar larvae were used for all experiments. For bioassays, the larvae were starved for one day before analysis.
Bioassay of the Toxin Using B. mori:
The LD50 of Cry2Aa toxin was determined with the mortality on day 7 using Probit analysis [32]. Five groups of B. mori larvae were bioassayed at five different concentrations of the toxin. Sucrose was added to the toxin solution to a final concentration of 6% (w/v), each larva was orally administered 20 μL of the toxin with a Pipetman and kept at 25 °C in the dark. The activity was calculated as described previously [30, 31].
Culture of Bt Strains and Isolation of Cry2Aa and Cry1Aa Crystals:
The a-spore gene-modified Bt, strain BT51, harboring the plasmid vector pBC16.1 encoding the cry2Aa9 gene [33] was cultured for Cry2Aa crystal production at 30 °C in Luria-Bertani medium for 72 h on a rotary shaker at 200 rpm in the dark. Tetracycline was added to the medium at a final concentration of 20 μg/mL to induce toxin production [33]. Two liters of the culture was centrifuged at 9,000 × g for 15 min at 4 °C and the pellet was suspended in 40 mL of 1 M NaCl. The suspension was sonicated at 20 kHz (VCX-130PB, Sonics & Materials, Inc., Newtown, CT, USA) for 5 min with 1-min intervals on ice after each 1-min sonication, and centrifuged at 15,000 × g for 10 min at 4 °C. The precipitate was washed, sonicated, and centrifuged three more times with the same NaCl solution.
Bt sotto T84A1 [34] was cultured in NYS medium in 500-mL bottom-baffled flasks to prepare Cry1Aa toxin as previously described [9], and the crystal was treated further as described above. Lastly, purified crystals of Cry2Aa and Cry1Aa toxins were suspended in double-distilled water at 1 mg/mL, respectively, and used in various experiments. Protein concentrations were measured with the Bradford method [35].
Alkali Solubilization of Cry2Aa Crystals:
Since Cry2Aa toxin was very resistant to alkali solubilization, the solubility of Cry2Aa crystals was checked. The crystal suspension of 1 mg toxin proteins/mL in 50 mM Na2CO3 (pH 10) containing 10 mM dithiothreitol (DTT) was prepared and incubated for 2 h at 37 °C. Each solubilization reaction was stopped by the addition of 1 M Tris-HCl (pH 8.3) containing 100 mM NaCl. The mixtures were centrifuged at 15,000 × g at 4 °C for 20 min, and the resulting supernatants and precipitates were recovered separately and dialyzed overnight against 50 mM Tris-HCl (pH 7.5) containing 50 mM NaCl using Seamless cellulose tubing (Viskase Sales Corp., Lombard, IL, USA).
Cry2Aa crystals were also incubated with 50 mM glycine NaOH buffer (pH 13) for 5 min. Solubilization was immediately ceased after the designated period by the addition of 1 M Tris, as described above. The resulting soluble and insoluble fractions were recovered by centrifugation, dialyzed as described above, and respectively applied to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Digestion and Purification of Cry2Aa Toxin:
Soluble Cry2Aa-60kD was digested with α-chymotrypsin (Pancreas, type II, Sigma Aldrich Chemicals, St. Louis, MO, USA) at a ratio of 50:1 (w/w) at 37 °C for 5 h with gentle reciprocal shaking. The digestion was ceased by the addition of phenylmethylsulfonyl fluoride (PMSF) at a final concentration of 0.1 mM and the digests were centrifuged at 15,000 × g at 4 °C for 20 min. The protein concentration of the recovered supernatant was adjusted to 2.3 mg protein/mL and sterilized by filtration through a membrane (Dismic-25HP, PTFE, 0.45 μm, Advantec Inc., Durham, NC, USA).
Two Milliliters of the sterilized digests containing 4.6 mg protein were applied to fast protein liquid chromatography (FPLC; AKTAFPLC, GE Healthcare Biosciences, Uppsala, Sweden) equipped with a HiLoad column (16/60, Superdex 200 pg, GE Healthcare Biosciences) loading with 120 mL of the buffer (50 mM Tris-HCl containing 50 mM NaCl). The effluent speed was 1.5 mL/min and 2 mL of each fraction was collected as described by Ohsawa et al. [30].
Preparation and Purification of Cry1Aa-60kD activated Toxin:
Crystals of Bt sotto T84A1 in 1 mL of the suspension containing 2 mg of protein were solubilized with 50 mM Na2CO3 (pH 10.5) containing 10 mM DTT for 2 h at 37 °C. The solubilized samples were dialyzed against 50 mM Tris-HCl (pH 8.3) overnight and the resulting suspension was centrifuged at 15,000 × g, 4 °C for 20 min. The supernatant was recovered as the soluble Cry1Aa protoxin. The resulting soluble protoxin was dialyzed using Seamless cellulose tubing.
Solubilized Cry1Aa protoxin was digested to a Cry1Aa-60kD with immobilized trypsin and the resulting toxin was purified with diethylaminoethyl Sepharose column chromatography using a linear gradient of 50–400 mM NaCl in 50 mM Tris-HCl (pH 8.3), as described previously [9]. The purified Cry1Aa-60kD was dialyzed against 50 mM Tri-HCl (pH 8.3) containing 50 mM NaCl and was further applied to FPLC as described above.
SDS-PAGE:
SDS-PAGE was performed as described by Laemmli [36]. A 20% volume of SDS-PAGE sample buffer was added to the sample and boiled for 3 min. Electrophoresis was performed at a constant current of 25 mA/gel plate with the standard mixture (Sigma Aldrich Chemicals), and proteins were stained with 0.2% Coomassie brilliant blue (CBB) R-250 (Sigma Aldrich Chemicals).
Western Blot Analysis of the Binding between Cry2Aa-60kD and -50kD, and B. mori BBM Proteins:
After SDS-PAGE, the proteins were blotted onto a polyvinylidene fluoride (PVDF) membrane (Hybond-P, GE Healthcare Biosciences) using a gel blotter with a 1.7 mA/cm2 membrane for 3 h. Subsequently, Western blotting was performed as described by Shitomi et al. [37] for the Cry2Aa-60kD and -50kD. Activated Cry1Aa toxin was also used to bridge the related data. The toxins were detected using rabbit antisera raised against Cry2Aa-60kD and Cry1Aa-60kD, respectively. The preparation of the antisera against Cry2Aa-60kD is described below. After three washes of the membrane with phosphate-buffered saline with Tween and 1% skim milk, the membrane was incubated with horseradish peroxidase-conjugated anti-rabbit IgG goat antibodies (Chemicon-Millipore Merck, Billerica, MA, USA) at a 20,000× dilution in phosphate-buffered saline with Tween and 1% skim milk for 1 h. The proteins were visualized using an Immobilon detection kit (Immobilon, Western Chemiluminescent, Millipore Merck, MA, USA) and LAS-3000 (Fuji Photo Film Co. Ltd., Tokyo, Japan).
Preparation BBMV from Midgut Epithelial Cells of B. mori:
One-day-old fifth-instar B. mori were paralyzed on ice, and the midgut was dissected and stored at –80 °C in METP buffer, pH 7.4 (300 mM mannitol, 5 mM EGTA, 24 mM MgCl2, 50 mM Tris-HCl, 1 mM PMSF) until use. After thawing the midgut, 1 mL of pre-chilled METP buffer per midgut was added and homogenized with a polytoron at 11,000 rpm (DIAX-900, Heidolph Elektro GmbH & Co., Germany). The BBMV was prepared from the homogenate by the method of Wolfersberger et al. [38], and the resulting sample was suspended with TEP buffer (20 mM Tris-HCl, 5 mM EGTA, 1 mM PMSF, pH 8.0).
Antisera Preparation:
After the SDS-PAGE and CBB staining, the Cry2Aa-60kD band was excised from the gel and the CBB was thoroughly rinsed off with distilled water. The gel was chilled and ground thoroughly with a mortar and pestle. SDS was added to the resulting gel suspension at 1% final concentration and shaken vigorously for 2 h with a reciprocal shaker to extract proteins. The supernatant was recovered by centrifugation, the remaining gel was extracted again with 1% SDS, and the supernatant was recovered with centrifugation as described above. This extraction was repeated three additional times, and all supernatants were pooled together, frozen with liquid nitrogen, and lyophilized overnight. Forty milliliters of 90% acetone were added to the lyophilized protein, shaken for 5 min at –40 °C, and the protein was precipitated by standing for 8 h at –40 °C. Acetone was decanted and thoroughly removed under a vacuum. The protein concentration was determined by the Bradford method [35]. To prepare antisera, Cry1Aa was also purified as described by Shitomi et al. [37].
Immunization of Rabbits with Cry2Aa-60kD and Cry1Aa-60kD:
Complete Freund adjuvant (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and Cry2Aa-60kD were mixed at a 1:1 ratio by volume, and a female Japanese White rabbit (3 kg) (SLC Co, Hamamatsu, Shizuoka, Japan) was immunized at the roots of the forefoot and neck. After a week, incomplete Freund adjuvant (Wako Pure Chemical Industries) was mixed with Cry2Aa toxin at 1:1 ratio by volume and the rabbit was immunized in the same manner as described above. The mixture was injected every other week for a total of three injections. One week later, whole blood was taken and allowed to stand for 3 h at room temperature and then left overnight at 4 °C. The specificity and strength of the antiserum were confirmed with Western blot analysis for Cry2Aa-60kD. Another rabbit was immunized with purified Cry1Aa-60kD.
Preparation of Cy3-labeled Cry2Aa-50kD:
One milligram of each Cry2Aa-60kD, -50kD and Cry1Aa-60kD were labeled with the fluorescent dye Cy3 (Mono-reactive Dye Pack, GE Healthcare Biosciences) according to the manufacturer instructions. Unbound Cy3 was removed with an Apollo 20-mL spin column (Orbital Biosciences, Topsfield, MA, USA).
Analyses of the Binding between Cy3-labeled Cry2Aa-60kD, -50kD and Cry1Aa-60kD, and the B. mori BBMV:
Cy3-labeled Cry2Aa-60kD, -50kD, and Cry1Aa-60kD were used in the binding assay. The three Cry toxins, each 250 μL, at final concentrations of 6.25, 12.5, 25, 50 and 100 nM, and 250 μL of the BBMV suspension corresponding to 1 mg protein/mL concentration were gently mixed for 1 h at room temperature, respectively. Each mixture was then centrifuged at 30,000 × g for 15 min at 4 °C, the supernatant was discarded, and the resulting precipitate was rinsed with 50 mM Tris-HCl (pH 7.5) containing 150 mM NaCl. The mixture was centrifuged again to remove unbound Cy3-labeled toxins. The rinsing step was repeated one more time. The final precipitate was suspended in 300 μL of Tris-HCl buffer, and the fluorescence intensity was determined by a fluorescence meter (RF-5300PC, Shimadzu Corp., Kyoto Japan) with excitation at 500 ± 15 nm and emission of 570 ± 15 nm.
In-gel Protein Digestion for Mass Spectra Analysis of BBM Proteins:
The protein bands visualized in the ligand blot analysis as described above were excised together with the gel to extract the proteins. The CBB dye was removed thoroughly from the gel with a mixture of 50% (v/v) methanol and 10% (v/v) acetic acid. Then, 100% acetonitrile was added to the gel to remove water and dried under a vacuum. One hundred microliters of reducing reagent, a mixture of 10 mM DTT and 25 mM ammonium bicarbonate, was added to the gel and allowed to stand for 45 min at 56 °C in a reaction tube with a tightly closed cap. The DTT was removed and 25 mM ammonium bicarbonate was added to remove any remaining DTT with shaking for 5 min.
Trypsin Digestion:
Thirty microliters of trypsin solution (20 ng/μL trypsin for mass spectrometry, Promega, Madison, WI, USA) in 25 mM ammonium bicarbonate was adsorbed to the excised gel. After removing the excess trypsin solution, 50 μL of 25 mM ammonium bicarbonate was added and allowed to stand overnight at 37 °C as reported previously [39, 40]. Trypsin reagents were then removed and 50 μL of 50% acetonitrile and 5% trifluoroacetic acid were added. The resulting peptides were extracted with thorough sonication and vortexing according to previous reports [39, 40]. The extraction was repeated, and the extracts were combined and analyzed by mass spectrometry.
Alkylation:
Protein alkylation was performed with a 100 μL mixture of 55 mM iodoacetamide and 25 mM ammonium bicarbonate by shaking for 30 min in the dark. The alkylation reagent was removed and further washed with a mixture of 100 μL of 50% acetonitrile and 25 mM ammonium bicarbonate for 5 min. This washing step was repeated five times according to previous reports [39, 40]. The gel was dried under a vacuum.
Matrix-assisted Laser Desorption Time-of-flight Mass Spectrometry (MALDI/TOF-MS) Analysis of the Trypsin Digest:
MALDI/TOF-MS analysis of the peptides was performed with the Autoflex III TOF/TOF system (Bruker Daltonics, Sweden) and analyzed with mass fingerprinting (Matrix Science; http://www.matrixscience.com/) using MTP/AnchorChipTM (600/384 TF, Bruker Daltonics, Solna, Sweden).
3. Results:
Solubilization of Cry2Aa Crystals by Alkali Solution at pH 10:
Cry2Aa and Cry1Aa crystals were respectively incubated at 37 °C for 2 h in 50 mM Na2CO3 solutions or 50 mM glycine NaOH, containing 10 mM DTT at final concentration of 1 mg toxin proteins/ml at pH 10. The resulting supernatant and precipitate were separated by centrifugation and applied to SDS-PAGE, respectively (Fig 1). Cry2Aa crystals were not solubilized in the glycine solutions with and without DTT at pH 10, and a dominant Cry2Aa single band was observed at around the 60 kDa zone on the SDS-PAGE gel from the insoluble fraction (Fig 1B, Lanes 3 and 5), whereas no clear bands were observed from the supernatant (Fig 1B, Lanes 2 and 4). In contrast, 86% of Cry1Aa was solubilized in the treatment with Na2CO3 at pH 10 containing 10 mM DTT, and a significant amount of the toxin was detected in the soluble fraction in the presence of DTT at around the 130-kDa zone (Fig 1A, Lane 4).
The toxicity of the Cry2Aa crystal were checked using 12, 4th instar B. mori larvae and the toxicity of LD50 of 0.0018 μg/larva was calculated with Probit analysis.
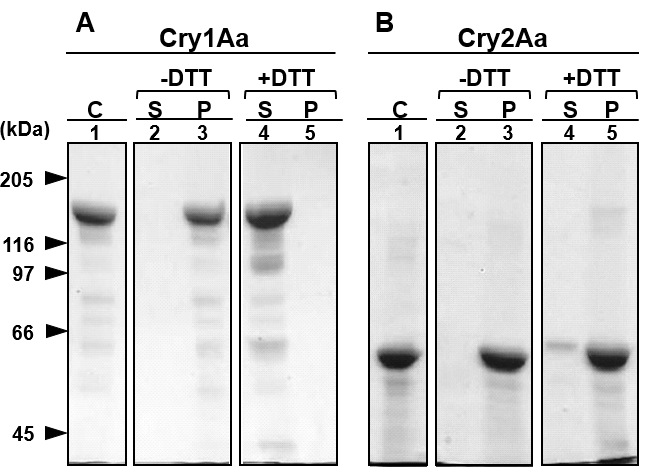
Figure 1: SDS-PAGE of Cry1Aa and Cry2Aa crystals solubilized with 50 mM Na2CO3, pH 10; The crystals of Cry1Aa (A) and Cry2Aa (B) were solubilized with 50 mM Na2CO3 and 50 mM glycine NaOH buffer at pH 10, respectively. Solubilization with Na2CO3 was performed with (+DTT) and without (-DTT) dithiothreitol. After the digestion, soluble (S) and insoluble fractions (P) were separated by a centrifugation at 15,000 × g for 20 min and applied to SDS-PAGE, respectively. The protein concentration in those fractions was determined with Bradford method. (C); Crystals were subjected to SDS-PAGE after solubilization with the sampling buffer at 95 oC.
Solubilization of Cry2Aa Crystals by Strong Alkali Solutions:
We further examined the effect of alkali solutions for the solubilization of Cry2Aa crystals using glycine-NaOH buffer at pH 8.3, 10, and 13 with and without trypsin (Fig 2). In the solubilization of the Cry2Aa crystals with pH 8.3 and 10, no solubilized protein bands were observed regardless of the presence of trypsin at either 30 and 60 min of incubations (Fig 2A and 2B). However, the buffer at pH 13 effectively solubilized the Cry2Aa crystals regardless of the presence of trypsin (Fig 2A and 2B, Lanes 5, 6, 9, and 10). Thus, trypsin did not enhance Cry2Aa crystal solubilization. Importantly, the Cry2Aa solubilized in these alkali conditions showed significant killing activity against 5th-instar larvae of B. mori 4 h after feeding (Table 1).
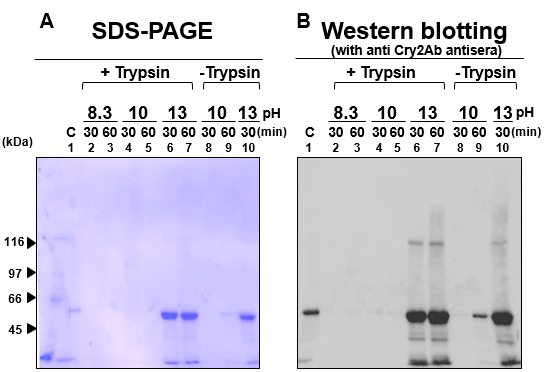
Figure 2: Solubilization of Cry2Aa crystals with strong alkali solutions; The Cry2Aa crystals were solubilized using glycine-NaOH buffers at pH 8.3, 10, and 13 with (+Trypsin), Lanes 2-7 or without (-Trypsin) trypsin, Lanes 8-10. Soluble and insoluble fractions were separated by a centrifugation and proteins in soluble fractions were subjected to the SDS-PAGE (A) with CBB staining and Western blotting (B) in which the proteins were detected with anti Cry2Aa-60kD antisera.
Moreover, the Western blotting patterns from SDS-PAGE of the 30- and 60-min digests at pH 13 were similar (Fig 2). Therefore, we further explored the efficacy of the pH 13 alkali solution to solubilize Cry2Aa in a shorter time. Cry2Aa crystals (13 μg) were solubilized with glycine-NaOH, pH 13, for 1–60 min at 37 °C and proteins recovered in the soluble fraction were analyzed with SDS-PAGE and Western blotting using antisera raised against Cry2Aa-50kD (Fig 3A, B). As expected, even with 1 min solubilization, more than 80% of the crystals were solubilized in the buffer (Fig 3A, B, Lane 1)
Prolonged incubation of 30 min at pH 13 reduced the amount of the solubilized Cry2Aa-60kD by 60%; however, the bioassay showed significant insecticidal activity 2–4 h after administration of the toxins (Table 1). This reduction was considered to reflect excess degradation of the toxin by the strong alkali solution.
| Table 1: Insecticidal activities of Cry2Aa solubilized under various conditions against the larvae of Bombyx mori. | ||||||||||||
| Test No. | Toxins | pH | Min. of | Temp. C | No. of larvae | Hour of feeding | ||||||
| incubation | used | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||||
| 1 | Cry2Aa* | H2O | 0 | 25 | 9 | 0 | 3 | 8 | 9 | 9 | 9 | 9 |
| 2 | Cry1Aa | H2O | 0 | 25 | 9 | 0 | 9 | 9 | 9 | 9 | 9 | 9 |
| 3 | Cry2Aa | 13** | 30 | 37 | 10 | 0 | 0 | 0 | 8 | 9 | 9 | 10 |
| 4 | Cry2Aa | 10*** | 30 | 60 | 10 | 0 | 0 | 6 | 6 | 8 | 9 | 10 |
| 5 | Cry2Aa | 10 | 10 | 100 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | Cry2Aa | 10 | 30 | 37 | 10 | 0 | 5 | 8 | 9 | 9 | 10 | ended |
| 7 | Cry2Aa | 10 | 60 | 37 | 10 | 0 | 4 | 6 | 6 | 9 | 10 | ended |
| *Crystals were prepared and suspended with water and orally admnistered to the larvae. | ||||||||||||
| **Glycine-NaOH buffer was used. | ||||||||||||
| ***Na2CO3-HCl buffer was used. | ||||||||||||
We were concerned about the potential inactivation of the toxin in such strong alkali conditions. To address this issue, the treatments were performed for only 1 min to 60 min at pH 13; in the shortest time period, 80% of the crystals by weight was solubilized (Fig 3) and the weight of the solubilized Cry2Aa crystals in the 30 min alkali treatment was approximately 60% of that of 1 min treatment (Fig 3). The digests from the 1 and the 5 min incubations were then applied to the bioassay, which demonstrated significant activity against B mori (Table 2).
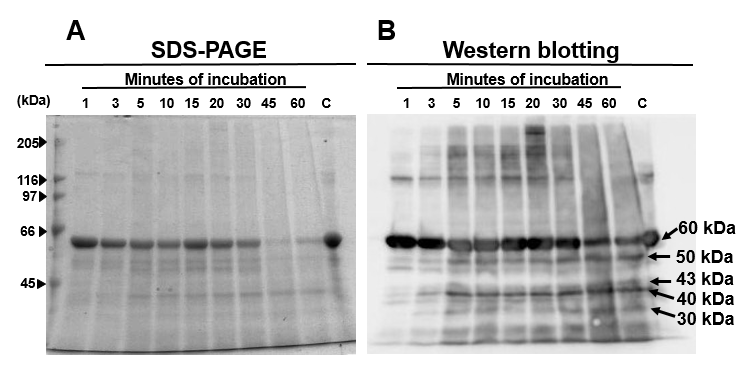
Figure 3: Solubilization of Cry2Aa crystals using glycine-NaOH buffer, pH 13; Cry2Aa crystals were solubilized with glycine-NaOH buffer at 37 °C for 1–60 min. Proteins in the soluble fraction were analyzed with SDS-PAGE (A) and Western blotting (B) using anti-Cry2Aa antisera raised against Cry2Aa activated toxin (Lane C). The Cry2Aa crystal (13.3 μg) was applied to the gel after treatment with the sample buffer from the PAGE gel kit
| Table 2: Insecticidal activity of purified Cry2Aa 50 kDa toxin against Bombyx mori | ||||||||||
| LD50 (μg/larvae)a) (95% confidence limit)b) | ||||||||||
| Cry2Aa 50 kDa toxin | Exp.1c), 1 min 0.022 (0.013~0.038) | |||||||||
| Exp.2d), 5 min 0.026 (0.014~0.042) | ||||||||||
| a) The toxins were administered via the oral rout to ten 5th instar larvae of B. mori. | ||||||||||
| b) 95% confidence limits were calculated with the Probit method. | ||||||||||
| c) and d) The Cry2Aa 50 kDa toxin used were solubilized with glycine-NaOH buffer, | ||||||||||
| pH 13 for 1 and 5 min, respectively, and soluble toxin proteins were purified with FPLC | ||||||||||
| as described in the text. | ||||||||||
a) The toxins were administered via the oral rout to ten 5th instar larvae of B. mori.
b) 95% confidence limits were calculated with the Probit method.
c) and d) The Cry2Aa 50 kDa toxin used were solubilized with glycine-NaOH buffer, pH 13 for 1 and 5 min, respectively, and soluble toxin proteins were purified with FPLC as described in the text.
Solubilization, Digestion, and Purification of Cry2Aa-60kD and -50kD:
Five minutes of incubation with the pH 13 buffer effectively solubilized the Cry2Aa crystals, and the activity was retained without loss compared with that of 1-min incubation (Table 2). Given that a 5-min incubation was much easier to control in the experiment, this condition was employed to solubilize Cry2Aa crystals.
After treatment of Cry2Aa crystals with the pH 13 buffer for 5 min, a single main peak was separated from the other several minor peaks at a loading volume of approximately 47 mL in FPLC (Fig 4A). This major peak of Cry2Aa-60kD was collected and further treated with α-chymotrypsin followed by chromatography.
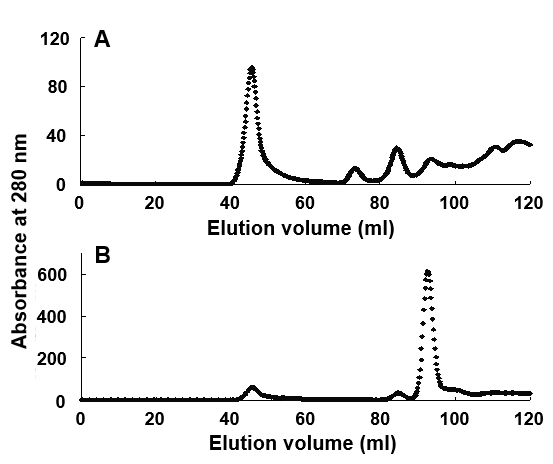
Figure 4: Separation of Cry2Aa 60 kDa protoxin and Cry2Aa 50 kDa toxin with fast protein liquid chromatography; Cry2Aa crystals were solubilized with the buffer at pH 13 for 5 min. The obtained major fractions were treated with α-chymotrypsin and subjected to chromatography. The proteins were monitored with ultraviolet absorbance at 280 nm (-♦-) and an elution volume of 43–45 mL (A) or 90–92 mL (B), representing the purified Cry2Aa 60 kDa protoxin and -50 kDa toxin, respectively.
A single major peak of Cry2Aa-50kD was separated at 94 mL from a few several minor peaks in FPLC (Fig 4B). The separated Cry2Aa-60kD and -50kD were collected at elution volume 41–51 mL (Fig 4A) and 90–96 mL (Fig 4B), respectively.
Confirmation of the Effectiveness of Purification of Cry2Aa- 60kD and Cry2Aa-50kD with FPLC:
The fractions of Cry2Aa-60kD and Cry2Aa-50kD recovered from FPLC were respectively analyzed with SDS-PAGE and Western blotting using anti Cry2Aa antisera. In SDS-PAGE, a clear 60 kDa protein appeared and no contamination of 50 kDa protein was found (Fig 5A, Lane 1). For the Cry2Aa-50kD, a single band appeared on the SDS-PAGE gel along with a thin band reflecting contamination of chymotrypsin (Fig 5A, Lane 2). Both toxins were blotted onto a PVDF membrane and detected with anti-Cry2Aa antisera, confirming positive expression of the Cry2Aa-60kD and -50kD with Western blotting (Fig 5B, Lanes 3 and 4). The clear separations of the Cry2Aa-60kD and -50kD with two treatments of FPLC confirmed the effectiveness of liquid chromatography subsequent to the alkali solubilization and chymotrypsin digestion.
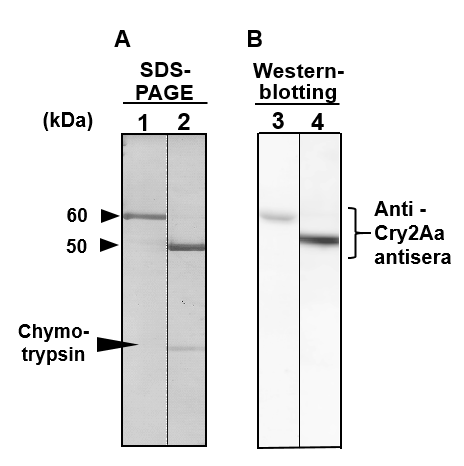
Figure 5: SDS-PAGE and Western blotting analyses of Cry2Aa 60 kDa protoxin and -50 kDa toxin purified with fast protein liquid chromatography; The purity of the Cry2Aa 60 kDa protoxin and -50 kDa toxin obtained from FPLC were estimated by SDS-PAGE (A, Lanes 1 and 2) with CBB staining and Western blotting (B, Lanes 3 and 4) using antibody raised against Cry2Aa 60 kDa protoxin.
Estimation of the Binding of Cry2Aa-60kD, -50kD and Cry1Aa-60kD to the BBMV of B. mori:
To estimate the binding affinity of the Cry2Aa-60kD and the -50kD, and the Cry1Aa-60kD to the B. mori BBMV, we determined the dissociation constant Kd. The toxins were visualized according to the labeled Cy3 fluorescence and each labeled toxin was independently mixed with BBMVs. The binding curves were constructed according to the fluorescence indicated an unbound and bound state to the BBMV, and corresponding Scatchard plots are shown in Fig 6. The Kd values between Cy3-labeled Cry2Aa-60kD, Cry2Aa-50kD and Cry1Aa-60kD with the B. mori BBMV are summarized in Table 3, demonstrating reduced binding for the Cry2a-50kD. These results suggested that the binding of BBMV with Cy3-Cry2Aa and Cy3-Cry1Aa occurred without any significant interference.
| Table 3: The dissociation constant (Kd) in the binding | |||||||
| between Cy3-labeled Cry2Aa and Cry1Aa toxins, and | |||||||
| Bombyx mori BBM | |||||||
| Cry toxins | Kd (nM) | ||||||
| Cry2Aa 60 kDa protoxin | 97.3 (89.1-110.5) | ||||||
| Cry2Aa 50 kDa toxin | 38.2 (30.8 - 42.4) | ||||||
| Cry1Aa 60kDa toxin | 80.8 (78.4 - 83.7) | ||||||
| The Kd values summarized here were calculated from | |||||||
| the Scatchard plots shown in Fig. 6. | |||||||
Ligand Blot Analyses of B. mori BBMV Proteins Bound to Cry2Aa-60kD, Cry2Aa-50kD and Cry1Aa-60kD:
Ligand blot analyses with the anti-Cry2Aa-60kD antisera clearly showed that the 68 and 60 kDa BBM proteins bound to both Cry2Aa-60kD and -50kD (Fig 7, Lanes 3 and 4), whereas Cry1Aa-60kD did not (Fig 7, Lane 2). Instead, Cry1Aa-60kD strongly bound to proteins that migrated to the zone corresponding to the size range of 100–250 kDa, where various BBMV proteins such as APNs and CadLPs migrated (Fig 7, Lane 2). The very thin band that appeared at around 60 kDa zone in Lane 2 was not the band specifically detected with ligand blotting; such thin bands or smears are occasionally observed in ligand blotting analyses.
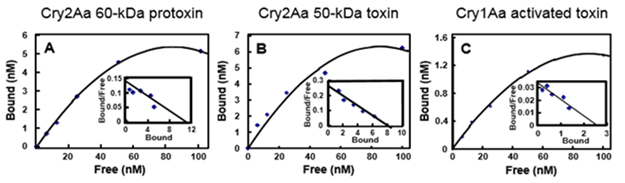
Figure 6: Binding of Cy3-labeled Cry2Aa 60 kDa protoxin, Cry2Aa 50 kDa toxin, and Cry1Aa 60 kDa toxin to Bombyx mori BBMV; The B. mori BBMV was mixed with Cy3-labeled Cry2Aa 60 kDa protoxin (A), Cry2Aa 50 kDa toxin (B), or Cry1Aa 60 kDa toxin (C) for 1 h in 50 mM Tris-HCl (pH 7.5) containing 150 mM NaCl. The fluorescence intensity of each Cy3-labeled toxin bound to the B. mori BBMV was determined with a fluorescence photometer at an excitation and emission wavelength of 550 ± 15 nm and 570 ± 15 nm, respectively. The inset graphs show Scatchard plots to determine the Kd values between Cy3-labeled Cry toxins used and the BBMV. The Kd values calculated from superimposed Scatchard plot are shown in Table 3. The Kd value was determined three times.
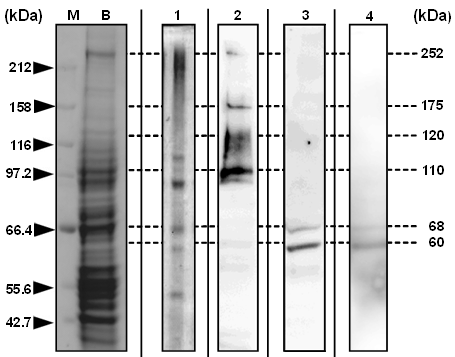
Figure 7: Western blotting of the binding of Cry2Aa 60 kDa protoxin, -50 kDa toxin, and Cry1Aa 60 kDa toxin with Bombyx mori BBM proteins; The proteins of the whole BBM were solubilized, applied to SDS-PAGE, and the protein migration pattern was visualized with CBB dye (Lane B). The proteins on the gel were blotted onto the PVDF membrane and visualized with CBB (Lane 1). The BBM proteins blotted onto PVDF were hybridized with Cry2Aa 50 kDa toxin, Cry2Aa 60 kDa protoxin, and Cry1Aa 60 kDa toxin. The toxins bound to BBM proteins were detected with the antisera raised against Cry1Aa 60 kDa toxin (Lane 2), Cry2Aa 50 kDa toxin (Lane 3), or Cry2Aa 60 kDa protoxin (Lane 4). Lane M: Protein marker.
MALDI/TOF-MS of the Bound 68 and 60 kDa Proteins from the BBM of B. mori:
The 68 and 60 kDa BBM proteins bound to Cry2Aa toxins (Fig 7) were recovered, processed, and applied to MALDI/TOF-MS for identification. The nine peptidyl fragments obtained from the 68 kDa BBM protein showed approximately 20% homology with v-type ATP synthase subunit A (Table 4A). In addition, the seven fragments obtained from the 60 kDa BBM protein showed 16% homology to ATP synthase subunit A (Table 4B). One fragment of the 60 kDa protein, corresponding to amino acid residues 209–235, showed homology to glycosyl hydrolase family 31 protein (Table 4C).
Table 4: Amino acid sequence of internal peptides from Bombyx mori 68 (A) and 60 kDa (B and C) BBMV proteins matching to those of vacuolar ATP synthase subunit A (A and B) and glycosyl hydrolase family 31 proteins (C).
|
A |
Position |
m/z |
Mr (expt) |
Mr (calc) |
Deduced amino acid sequence |
|
|
|
46-57 |
1361.7547 |
1360.7474 |
1360.7350 |
R.VGYNELVGEIIR.L |
|
|
|
58-83 |
2679.2950 |
2678.2877 |
2678.3109 |
R.LEGDMATIQVYEETSGVTVGDPVLR.T |
|
|
|
187-200 |
1608.7863 |
1607.7790 |
1607.7679 |
K.VTDVVLETEFDGER.Q |
|
|
|
203-213 |
1294.6594 |
1293.6521 |
1293.6540 |
K.YSMLQVWPVR.Q + Oxidation (M) |
|
|
|
324-338 |
1749.8837 |
1748.8765 |
1748.8621 |
R.EASIYTGITLSEYFR.D |
|
|
|
428-436 |
1134.0630 |
1133.5990 |
1133.5869 |
K.NYPEFVPLR.T |
|
|
|
514-530 |
2092.0060 |
2090.9987 |
2090.9909 |
K.LLKDDFLQQNSYSSYDR.F |
|
|
|
543-552 |
1259.6114 |
1258.6041 |
1258.6016 |
K.NIITFYDMSR.H |
|
|
|
599-613 |
1728.8026 |
1727.7954 |
1727.7825 |
K.ADFDQLLEDMSAAFR.N |
|
|
|
|
|
|
|
|
|
|
B |
Position |
m/z |
Mr (expt) |
Mr (calc) |
Deduced amino acid sequence |
|
|
|
46-57 |
1361.7429 |
1360.7356 |
1360.7350 |
R.VGYNELVGEIIR.L |
|
|
|
83-109 |
2692.5722 |
2691.5650 |
2691.5323 |
R.TGKPLSVELGPGILGSIFDGIQRPLK.D |
|
|
|
187-200 |
1608.8052 |
1607.7979 |
1607.7679 |
K.VTDVVLETEFDGER.Q |
|
|
|
203-213 |
1278.6575 |
1277.6502 |
1277.6591 |
K.YSMLQVWPVR.Q |
|
|
|
324-338 |
1749.9063 |
1748.8991 |
1748.8621 |
R.EASIYTGITLSEYFR.D |
|
|
|
428-436 |
1134.6304 |
1133.6231 |
1133.5869 |
K.NYPEFVPLR.T |
|
|
|
599-613 |
1728.8122 |
1727.8049 |
1727.7825 |
K.ADFDQLLEDMSAAFR.N |
|
|
|
|
|
|
|
|
|
|
C |
Position |
m/z |
Mr (expt) |
Mr (calc) |
Deduced amino acid sequence |
|
|
|
*209-235 |
3190.5026 |
3189.4953 |
3189.4237 |
K.DTGFPNAQFEIDDLWEICYGSLTVDER.K |
|
m/z means experimental m/z values, Mr (expt) means relative molecular mass values transformed from m/z and Mr (calc)
molecular masses calculated from the matched peptide sequence. Deduced amino acid sequences indicate the corresponding fragments of vacuolar ATP synthase subunit A (A and B) and glycosyl hydrolase family 31 protein (C*)
Discussion:
Many genetically modified plants have been generated with Bt Cry1A genes or a combination of Cry1A and other Bt genes [41], therefore, continuous selection pressure from the Cry1A toxins has led to the emergence of resistant insects [15, 16, 42]. To overcome this threat, alternating the use of Cry1A and Cry2A, which can kill some Cry1A-resistant insects, has been proposed [17]. This strategy has been realized in the GM cotton Bollgard II expressing Cry1Ac and Cry2Ab, developed by Monsanto.
Cry1Aa kills Lepidoptera only, whereas Cry2Aa kills both lepidopteran and dipteran insects [43], which is attributed to the different binding sites from each other in the midgut epithelial cell membrane. Therefore, Cry2A is an important foreign gene to expand the resistance spectra in GM plants and to enhance the efficacy of spray formulations.
In previous study [30], we showed that the Cry2Aa-50kD segregated at the N-terminal 49 amino acid residues from the Cry2Aa-60kD, which corresponded to the loss of 114 amino acid residues at the N terminus. Nevertheless, Cry2Aa-50kD showed more intense toxicity against L. dispar and B. mori compared with that of the Cry2Aa protoxin. The activity against L. dispar was two-fold higher than that of the protoxin. Thus, it was suggested that the α-helix0-α-helix3 region of Cry2Aa was not necessary for its insecticidal activity [30]. If this is true, the region in domain I of the Cry2Aa, which was removed during the activation processing, may interfere the binding between the toxin and binding epitope(s), as suggested by Morse et al. [21].
In this study, we found that the Kd between the B. mori BBMV and Cry2Aa-50kD was slightly lower than that between Cry2Aa-60kD and the BBMV. Thus, it is possible that the fully exposed region in the toxin contains an epitope for binding with the BBMV, resulting in more higher binding affinity. The Kd values for the binding between Cry1Aa and the B. mori BBMV were almost identical to the values determined previously in our laboratory [9, 37, 44], suggesting that the experimental system itself was reliable. Although the Kd value of 38.2 nM for the binding between Cry2Aa-50kD and BBMV we determined was sufficiently low to suggest that the binding likely occurs under physiological conditions. But still we need to evidence the binding between the 68 or 60 kDa BBM proteins and Cry2Aa-50kD is taking place under the physiological condition on the BBM.
The binding between Cry2Aa or Cry1Aa toxins and solubilized BBM proteins was explored using Western blotting with corresponding antisera and it showed that the 60 and 68 kDa BBM proteins bind to the toxin, but BBM proteins migrated to the 110–250 kDa zone in the SDS-PAGE did not bound to Cry2Aa. Thus, the two toxins did not bind to the same BBM proteins. According to various reports, these BBM proteins detected at 110–250 kDa likely include receptor proteins for Cry1A toxins, such as APN [7, 37, 45], CadLP [46], and the chlorophyll-binding protein P252 [10]. Our results indicating that Cry2Aa toxins may not bind to these proteins, strongly support the idea that the BBM proteins binding with the Cry2Aa toxins may be different from those reported as the binding proteins for Cry1A toxins.
Other interesting aspect is the solubility of the Cry2Aa crystal in alkali solution, thus as shown in Fig 1, the solubility of Cry2Aa at pH 10 was very low with and without DTT. This was completely different from that the solubilization of Cry1Aa crystals. Initially, we thought that this difference between the both Crystals likely reflects the disulfide-bond content in the crystal protein and/or protein folding in the genetically modified a-spore Bt strain BT51 harboring vector pBC16.1 encoding the cry2Aa9 gene. However, already these characteristics of the Cry2Aa behavior in the alkaline solution were reported in the wild type Bt. The toxicity of Cry2Aa solubilized with a strong alkali, pH 13, was destroyed the activity with prolonged incubation. However, solubilization was effective in a very short incubation period; the solubilized Cry2Aa crystals with the incubation of 1 or 5 min in the strong alkali exhibited approximately one-tenth or one-fifteenth of the activity of the crystals. The LC50 of the purified crystals of Cry2Aa alkali-solubilized for 1 min and 5 min against the B. mori larvae were strong and nearly identical (Table 2).
Tojo and Aizawa [47] reported a dramatic increase in the solubility of Cry1A and Cry2A crystals of Bt HD-1 with pH 13 alkali treatment in B. mori toxin. We also confirmed the effectiveness of the alkali treatment and fragmentation with Western blotting using anti-Cry2Aa antisera. The analysis with the antisera suggests that the determination of fragmentation using antisera must be necessary and effective to elucidate the activation mechanism of Cry2Aa crystals in the midgut. When Tojo and Aizawa [47] digested the Cry2Aa crystals with B. mori midgut juice, protein fragments of around 50, 43, and 40 kDa appeared in SDS-PAGE. They concluded that these proteins likely reflected the content of the B. mori midgut juice. We digested the Cry2Aa crystals with pH 13 alkali treatment and found clear protein bands at 57, 50, 43, 40, and 30 kDa; among them, 57, 43, and 30 kDa fragments were positive in the Western blot analysis. As a conclusion, we are thinking that the alkali solubilization at pH 10 in the midgut will produce a small amount of Cry2Aa fragment having a strong insecticidal activity. Even in pH 10, like in the insect midgut, some active fragments obtained are enough to kill the toxin susceptible insect. Alternatively, the alkali treatment at pH 13 with a very short period of 1 or 5 min incubation will work to obtain a large amount soluble and active 50 kDa Cry2Aa toxin.
Audtho et al. [48] digested Cry2Aa with the midgut fluid from L. dispar and found 58 and 49 kDa peptides. Although the 58 kDa peptide disappeared after further digestion for 60 min, the 49 kDa peptide remained. But interestingly it lost toxicity against L. dispar. These very steady efforts obviously can help to resolve the mechanism of the processes required to produce active Cry2Aa toxins in diverse insect groups. This implies another idea that solubilization of a very small parts of the Cry crystals will be enough to kill the susceptible insects in some cases as we mentioned above.
In our current studies, the 60 and 68 kDa BBM proteins were suggested to be the protein(s) of the Cry2Aa toxin. Homology analysis with MALDI/TOF-MS showed the peptides derived from these two peptides had homology to v-type ATP synthase subunit A. This finding is in line with previous studies. In H. virescens larvae, ATPase was found to bind to Cry1Ac, and the expression of ATPase was higher in Cry1Ac-resistant Plodia interpunctella [26]. The v-type ATPase subunit B bound to Cry1Ac in H. armigera, along with actin and heat shock protein [27]. In A. aegypti larvae, ATPase also bound to Cry4Ba [25]. In another study, A. aegypti larvae became hypersensitive to Cry11Aa when ATP synthase subunit B expression was inhibited by RNA interference, and the authors found a resistant phenotype of A. aegypti when they silenced the heat shock protein [49].
Several recent studies have provided new insight into receptor proteins of Cry toxins. Onofre et al. [50] showed that Cry2Ab does not share binding sites with Cry1Ab toxin in the Manduca sexta BBMV. They also showed that Cry2Ab did not bind to ALP or CadLP but instead bound to APN2 in M. sexta based on cloning and ligand blot experiments. In contrast to our results, Zhao et al. [29] demonstrated the binding of Cry2Aa to CadLP, APN4, and ALP in H. armigera. They also found a much greater reduction in the toxicity of Cry2Aa when APN4 expression was silenced compared to when ALP2 or CadLP was silenced. Qiu et al. [28] reported that Cry2Aa bound to v-ATPase subunit B of S. exigua, and knockdown of the subunit B gene significantly decreased the susceptibility of the insect larvae to the toxin. Alternatively, Yuan et al. [51] reported that ALP2 was the binding protein for Cry2Aa in S. exigua. Although these various reports show some conflicting results along with several inconsistencies, they nevertheless offer insight and targets for developing more precise and specific experiments in the future. Several possibilities can be proposed to explain these discrepancies in light of previous results and the new findings of the present study.
First, the binding between ATP synthase and Cry toxin appears to be a more common phenomenon than previously considered; some basic knowledge of v-type ATP synthase can provide insight into these findings. V-type ATP synthase is a proton pump that is widely distributed in all organisms [52, 53]; that locating in the gut of various insects is a typical H+ pump, which intakes K+ and releases H+ outside the cell [54, 55]. ATP synthase is considered to originate from archaebacteria [56] and evolved into various proteins involved in energy generation in insects. Therefore, if the epitopes recognizing Cry toxin that were acquired in archaebacteria were incorporated into subsequent generations, they may have spread into other proteins, deviating from their original function in energy synthesis. Thus, a homology search should be performed over as wide a region of the target protein as possible to confirm that the target protein is involved in a function related to energy generation.
Second, caution is required when interpreting the data obtained from experiments involving the interference of ATP synthase. Since ATP synthase is one of the most important enzymes for cellular activities, its knockdown inevitably causes a multitude of severe changes throughout the cells at different time spans. In other words, the side effects derived from the knockdown of these essential enzymes must be carefully distinguished from the targeted effect under consideration.
Candas et al. [57] offer some interesting insight into this aspect. They found that the expression of v-type ATP synthase in a Cry toxin-resistant strain of Plodia interpunctella was higher than that of a susceptible strain. This could be a compensatory mechanism to overcome the malfunction of the ATP-generating system caused by Cry toxins. Xia et al. [58] also explored the binding between v-type ATPase subunit B and Cry1Ac in P. xylostella and found that the level of the ATPase was significantly higher in the Bt-resistant P. xylostella strain. Thus, it would be interesting to evaluate whether the binding of Cry2A toxin would disturb the flux of H+/K+ transport in the midgut BBMV [59].
Third, we must consider the topology of the BBMV isolated from the midgut epithelial tissue. When membranous vesicles are isolated from the cell membrane fraction, the topology might be changed to a certain degree. Cell organelles such as the mitochondria, endoplasmic reticulum, and also potentially the BBMV composed of membranes with a curvature have a tendency to turn inside out according to changes in the tension of the membrane. Thus, during preparation of the BBMV using the method of Wolfersberger et al. [38], a certain amount of the BBMV will likely form a vesicle in which the inner membrane is turned out according to a pressure change.
Fourth, and arguably the most important, point is consideration of the location of ATP synthase. These enzymes are generally localized on the surface of the inner membranes of cells or organelles; therefore, for effective binding, Cry 60 kDa toxin must be able to deeply penetrate the membrane to access the subunit of ATP synthase. This direct interaction requires the long peptidyl portion of the Cry 60 kDa active toxin to reach the site. In the so-called pore-forming theory, a few α-helices with short lengths generally penetrate the cell membrane to form hexagonal structures through which the efflux/influx of ions occurs. Two interesting results showing the possibility to overcome the disadvantage of the pore-forming theory were proposed by Tomimoto et al. [60] and Nair and Dean [61], respectively. They reported that most of the Cry1Aa-60kD toxin molecule penetrated the BBMV. If this is also the case for Cry2Aa, it is possible to speculate that some parts of the Cry2Aa toxin interact with the inner cellular subunits of the ATP synthase. Gazit et al. [62] suggested that the loop of Cry toxin connecting α4 and α5 may interact with the inner leaflet of the membrane. In this case, the active 50–60kD Cry toxin molecules may be able to interact with proteins localized on the surface of the inner cell membrane.
Fifth, as mentioned above, Zhao et al. [29] demonstrated the binding of Cry2Aa to CadLP, APN4, and ALP in H. armigera. It is important to recognize that insects exhibit unique patterns of parallel evolution throughout their long history; thus, lepidopteran insects belonging to different groups have distinct evolutionary histories. H. armigera belongs to the Noctuidae, whereas B. mori is a member of the Bombycidae; accordingly, caution must be exercised when generalizing a result obtained from a specific insect to a certain group at large.
Recently, Cry toxin was reported to bind with the ABCC and various members of the ABCC superfamily, involving in the transportation and secretion of various biotic materials such as dyes and drugs, are suggested to be true receptors for Cry2Ab [11] and Cry1Ac toxins [12] in some lepidopteran insects. The ABCC has two transmembrane regions separated by an ATP-binding site, and there is another binding site located around the carboxy-terminal. The amino- and carboxy-termini of ABCC protein and the two ATP-binding sites are located on the internal surface of the cell membrane. However, the Cry toxin binding site is located on the outside of the cell membrane to enable easy binding to the site. When ABCC was mutated to alter the binding of Cry toxin, the insect cell line became either resistant or susceptible to Cry toxin depending on the site of the mutation [13]. Thus, the ABCC indeed appears to be one of the true receptors for Cry toxin. Nevertheless, the data available related to Cry2A remain insufficient to generalize the mechanism compared with the more extensive research performed on Cry1A [14]. In fact, recently several reports showed the data containing the discrepancy to these results and many other reports are showing very interesting results saying that ABCC is not main single player in the Cry toxins in various relationships between plant and insects [63-68] in different science fields such as developing biopesticide using double stranded RNA of the v-ATPase. Overall, the complex relationships between Cry toxin and ABCC in each insect are likely due to the divergent characteristic parallel evolution of insects. Therefore, consideration of all of the aspects raised above is needed to generalize the results obtained from different insects.
In this study, we identified that one of the internal fragments of the 60 kDa peptide of the B. mori BBM protein showed homology with glycosyl hydrolase family 31 protein (Table 4, Lower, C, amino acids 209–235). Van-Munster et al. [69] and Rodríguez-Cabrera et al. [70] also reported that S. frugiperda treated with the Cry1Ca toxin showed a reduction in the expression of glycosyl hydrolase family 31 protein. Therefore, this finding warrants further investigation. These knowledges can ultimately help to improve the effectiveness of Bt toxins and overcome resistance in each insect.