Agricultural Research Pesticides and Biofertilizers
OPEN ACCESS | Volume 6 - Issue 1 - 2026
ISSN No: 2994-0109 | Journal DOI: 10.61148/2994-0109/ARPB
Akinrotimi O.A1,3*, Wilfred–Ekprikpo P. C2 and Ukwe O.I.K3
1African Regional Aquaculture Center/Nigerian Institute for Oceanography and Marine Research, Buguma, P.M.B. 5122, Port Harcourt, Rivers State, Nigeria
2Aquaculture Department , Nigerian Institute for Oceanography and Marine Research, 3, Wilmot Point Road, Victoria Island, PMB 12729, Lagos – Nigeria
3Department of Fisheries and Aquatic Environment, Faculty of Agriculture, Rivers State University, Nkpolu-Oroworukwo, Port Harcourt, Rivers State, Nigeria
*Corresponding Author: Akinrotimi O.A, African Regional Aquaculture Center/Nigerian Institute for Oceanography and Marine Research, Buguma, P.M.B. 5122, Port Harcourt, Rivers State, Nigeria.
Received: June 08, 2021
Accepted: June 14, 2021
Published: June 21, 2021
Citation: Akinrotimi O.A, Wilfred–Ekprikpo P. C and Ukwe O.I.K. (2021) “Changes in Lymphocytes in Three Sizes of African Catfish (Clarias gariepinus) Exposed to Different Chemicals in the Laboratory.”, Journal of Agricultural Research Pesticides and Biofertilizers, 2(1); DOI:http;//doi.org/06.2021/1.1027.
Copyright: © 2021 Akinrotimi O.A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Changes in the number of lymphocytes in the blood of three sizes of Clarias gariepinus exposed to three pesticides paraquat dichloride (PARAQ), 2,2-dichlorovinyl phosphate (DDVP) and dimethoate (DMC) at the concentrations of 0.00 control, 1.00, 2.00, 3.00 and 4.00 mg/L were carried out. The result obtained indicated a concentration dependent increase which was more pronounced in the fish exposed to paraquat dichloride, followed by DDVP, while the lowest effect was observed in the fish exposed to dimethoate in all sizes of the fish. The result revealed that paraquat was more toxic to the fish, causing more stress as indicated through elevation of the lymphocytes in the blood stream of the fish.
1. Introduction:
Pesticides are known to cause severe environmental problems, especially in the aquatic environment. This phenomenon is more pronounced during the dry season, because during this period, the dilution capacity of the water systems is low, thus increasing the risk of high concentrations of toxic chemicals. Moreover, the dry season is often the critical period for many animals, especially fish and birds [1,2]. Water pollution by pesticides enters aquatic environment mostly through run offs from agriculture activities. These can lead to fish mortality, reduced fish productivity or elevated concentrations of undesirable chemicals in edible fish tissue, which are detrimental to the health of humans consuming these fishes [3,4].
Analysis of blood is important in the field of fisheries research, especially in the area of toxicology, health management and environmental monitoring [5]. Haematological parameters are very critical indices used in assessment of physiological changes in fish when compared to the control values. These alterations depend on species, age, sex and exposure of fish to diseases [6]. In both terrestrial and aquatic animals, variations in the blood indices of fish, which take place consequent of some damages in a number of tissues or organs, can be utilized to ascertain and validate the dysfunction or injuries of the latter (organs or tissues). [7]. However, in fish, these parameters are more related to the response of the defensive mechanism in fish. Although the defensive mechanisms of fish to xenobiotics have been investigated, it is obvious that species differences of these mechanisms exist. Thus, evaluation of leucocytes in response to contaminants is crucial in toxicological studies in fish.
One of the important leucocytes commonly assessed in fish is lymphocytes. Lymphocyte can be described as one of the major units of white blood cell and differential counts in an animal body defense structure [8]. Lymphocytes consists of inherent destroyers cells (that play key role in cell-mediated, cytotoxic intrinsic resistance), T cells (for cell decided, cytotoxic adaptive immunity), and B cells (for humoral, antibody-motivated adaptive defense ). They are the mjor categories of cell originated in lymph, which prompted the name "lymphocyte”. Lymphocytes are the key effectors cells of adaptive immunity [9]. For activation, lymphocytes require exposure to self and non-self-antigens in the context of major histocompatibility complex (MHC) molecules. Antigen–MHC complexes are presented to lymphocytes on the surface of antigen presenting cells [10].
Many authors have reported the effect of diverse pesticides on the behaviors and haematological responses in different species of fish, [11-13], and have found varying responses after exposing the fish to varying sublethal concentrations of toxicants. The intention of this work is to evaluate and add to knowledge on the variations in arrangements of lymphocytes structures in various sizes of C. gariepinus treated with three chemicals in the laboratory.
2.Materials and Methods:
Experimental Fish:
Seventy five each of juveniles (mean length 13.54±1.78 cm; mean weight 100.65±2.89g), sub adults (mean length 17.99±1.64 cm; mean weight 400.98±2.07g) and adults (mean length 27.08±2. 02cm; mean weight 1000.04±2.88g) of C. gariepinus were purchased from a commercial farm in Port Harcourt, Nigeria. The fishes were transported in six open 50l plastic containers to the African Regional Aquaculture Center Aluu, Port Harcourt and acclimated for a period of seven days.
Preparation of Test Solutions and Exposure of Fish:
Three variants of chemicals namely: paraquat dichloride (PARAQ), 2,2-dichlorovinyl phosphate (DDVP) and dimethoate (DMC) utilized in this trial were procured from a chemical shop in the city of Port Harcourt, Nigeria. Three sizes of C. gariepinus were exposed to each of the chemical at the concentrations of 0.00 control, 1.00, 2.00, 3.00 and 4.00 mg/L in triplicates. Five specimens of C.gariepinus were distributed at random into each of the experimental container. The experiment took place for a period of three weeks. The water in the experimental container was exchanged daily. The fish were fed twice daily at 3% body weight with a commercial feed.
Haematological Studies:
Haematological analysis was done at the end of the trial. The fishes were taken out separately from the experimental tank by using a small scoop net and placed its belly upward on a table. Blood samples of about 2 mL were collected from the caudal peduncle (Stoskopf, 1993) with the aid of a 5 mL plastic syringe, 2 mL of the blood was dispensed into Ethylene Diamine Tetra-acetic Acid (EDTA) anticoagulant for haematological studies .Leukocyte count (WBC) were determined using the improved Neubauer haemocytometer after appropriately diluted. Differential leukocyte counts were determined by scanning Giemsa’s stained slides in the classic manner [14].
Statistical Analysis:
The results obtained from the study were collated and were analyzed by using SPSS 22.0 version. A one-way analysis of variance (ANOVA) was used to evaluate the differences between the means. Duncan multiple range test (Duncan, 1955) was further used to separate the mean differences at 0.05 significant levels.
3. Results:
The effects of paraquat dichloride (PARAQ), 2,2-dichlorovinyl phosphate (DDVP) and dimethoate (DMC) on the lymphocytes of juveniles, sub adults and adult sizes of C.gariepinu are shown in Figures 1,2 and 3 respectively. The result indicated that the lymphocytes levels in the treatment groups were significantly higher (P < 0.05) than the control in all sizes. These increments in all the sizes of fish exposed to the chemical, progressively increased with increasing concentrations of the chemicals. In this study, the highest values of lymphocytes was observed in the fish exposed to paraquat, while the lowest was in dimethoate (DMC) in all sizes of C.gariepinus.
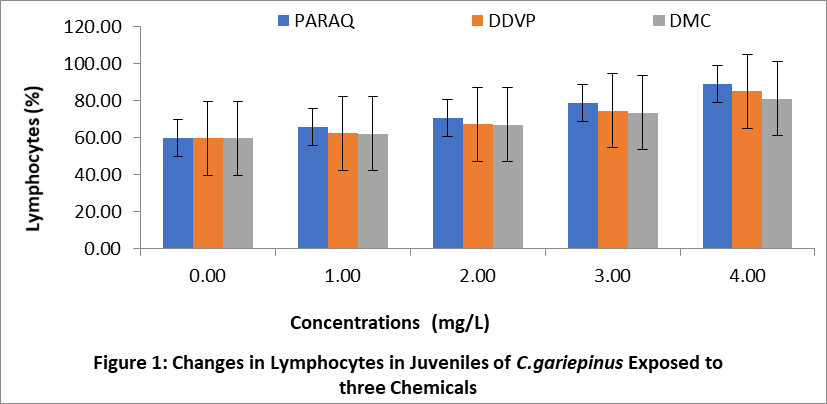
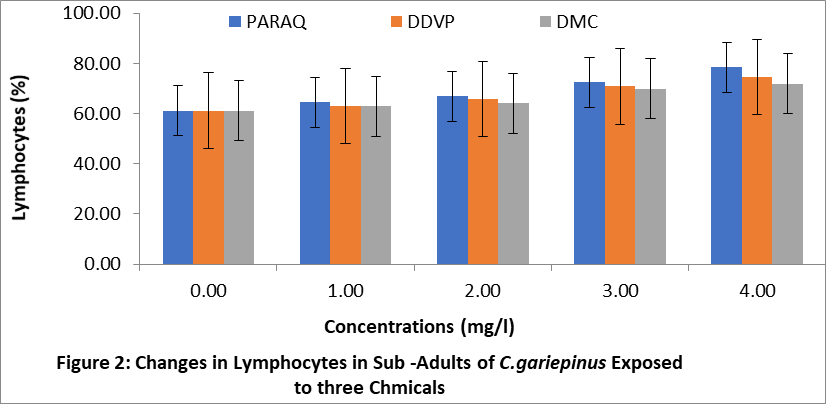
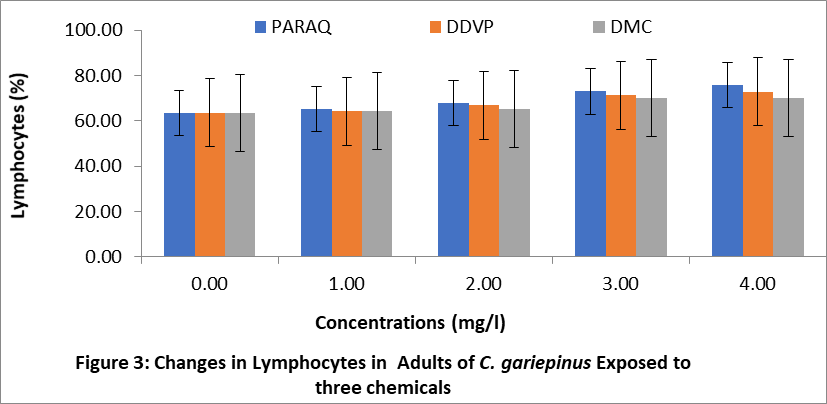
4. Discussion:
Fish exposed to environmental contaminants usually show evidence of physiological distortions such as disturbances in blood stability, ion regulator and oxygen uptake. This becomes noticeable, since blood is an indicator of the physiological condition of animals. Analyses of blood indices are essential in monitoring the physiological condition of fish and as a pointer of the wellbeing of fish in aquatic environment. Blood parameters are frequently considered when clinical analysis of fish physiology is applied to determine the sublethal concentration effects of toxicants both in the wild and laboratory. In this study, effects of pesticides on lymphocytes were observed, due to the fact that differential counts are sensitive to application of pesticides [14]. One of the most elementary ways to assess the immune system is to explore changes in the white blood cells count and its types such as lymphocytes [15].
The lymphocytes are reported to be responsible for immune response [16]. The nucleus takes over the whole of the cell, leaving only a constricted perimeter of basophilic cytoplasm in which there are only some mitochondria and segregated ribosomes. The number of lymphocytes in the blood is noticeably greater in fishes than in mammals [17]. Significant increase in lymphocytes concentration in fish exposed to different concentrations of pesticides was observed in this study. High levels of lymphocytes count indicate damage due to infection of body tissues and severe physical stress. Similar findings were observed in fish exposed to toxicants in the laboratory [18]. Also, Akinrotimi et al. [19], documented significantly higher values of lymphocytes in fish exposed to higher industrial effluents concentrations. In this assay, exposure of different sizes of C. gariepinus to different pesticides concentrations caused leukocytosis, characterized by higher number of lymphocytes. Leukocytosis has been attributed to an increase in leukocyte to protect the organism against infections in pesticides -damaged tissue [20].
5. Conclusion:
Toxicological and environmental issues resulting from the widespread use of pesticides in agriculture have raised concerns, particularly with respect to the potential toxic effects in humans and animals. The exposure of C.gariepinus to paraquat dichloride (PARAQ), 2,2-dichlorovinyl phosphate (DDVP) and dimethoate (DMC) were associated with alterations in lymphocytes levels, resulting in stress to the organism. These pesticides are therefore classified as belonging to substances toxic for fish. Long-term exposure to these chemicals can affect immunity profiles of C.gariepinus.