Agricultural Research Pesticides and Biofertilizers
OPEN ACCESS | Volume 6 - Issue 1 - 2026
ISSN No: 2994-0109 | Journal DOI: 10.61148/2994-0109/ARPB
Wilfred–Ekprikpo P.C
Aquaculture Department, Nigerian Institute for Oceanography and Marine Research, 3, Wilmot Point Road, Victoria Island, PMB 12729, Lagos, Nigeria.
Corresponding Author: Wilfred–Ekprikpo P.C, Aquaculture Department, Nigerian Institute for Oceanography and Marine Research, 3, Wilmot Point Road, Victoria Island, PMB 12729, Lagos, Nigeria.
Received: May 05, 2021
Accepted: May 10, 2021
Published: May 17, 2021
Citation: Wilfred–Ekprikpo P.C. (2021) “Changes in Electrolytes in Heterobranchus longifilis Exposed to Sub Lethal levels of Different Chemicals in the Laboratory”, Journal of Agricultural Research Pesticides and Biofertilizers, 1(2); DOI:http;//doi.org/05.2021/1.1006.
Copyright: © 2021 Wilfred–Ekprikpo P.C. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Changes in some electrolytes which include Sodium (Na+), Calcium (Ca2+), Potassium (K+), and Chloride (Cl-) in the plasma of Heterobranchus longifilis exposed to three different chemicals: paraquat dichloride (PARAQ), 2,2-dichlorovinyl phosphate (DDVP) and dimethoate (DMC) at different concentrations of 0.00 control, 0.05, 0.10, 0.15, 0.20 and 0.25 mg/L were carried out to determine the levels of osmotic impairment on fish exposed to these chemicals. A total of 150 juveniles sizes of Heterobranchus longifilis were used for the study. Results from the study indicated that the values of Na+ and K+ ions significantly increased (P < 0.05) in the exposed fish when compared to the control values. However, significant reduction (P < 0.05) of Ca2+ was equally observed in the exposed fish. However, the values of Cl- between the control and exposed fish were within the same range. Comparatively, these alterations were more pronounced in the fish exposed to paraquat dichloride, followed by DDVP and least in the fish exposed to dimethoate.
Introduction:
In recent years increasing rate of population, industrialization, urbanization and agricultural activities have become veritable sources of water pollution all over the world [1]. Majority of these contaminants find their way into the aquatic environment through diverse sources such as runoffs and discharge of wastes from industrial and domestic sources [2]. One of the chemicals being used today in homes and agricultural field is pesticides. According to Gabriel et al. [3], pesticides can be described as a diverse range of chemicals with differences in their mode of action, uptake by the body, metabolism and elimination from the body and toxicity to target and non-target organisms. Potential risks of pesticides in urban and rural centers mostly depend on dose, toxicity level, exposure period and sensitivity of the organism to the contaminants [4].
In the aquatic environment, several organisms are known to concentrate or accumulate toxic substances from their surroundings without any apparent damage to themselves and therefore act as toxicant depositors, making the toxicants available to predators when they are consumed.[5].Myriads of issues of the concomitant unpleasant effects of contaminants on fish and its consumers have been reported by several authors {6,7,8].Conversely, aquatic organism are exposed to long-term environmental stress, consequent of their exposure to sublethal concentrations of contaminants such as pesticides. On the other hand, in the long run analysis, these sublethal concentrations may have harmful effect on organisms in the aquatic environment such as lethal concentrations [9]. Sublethal concentrations of pollutant in aquatic environment have been reported to have a drastic effect on the behavioural pattern of fish and its physiology such as electrolytes [10, 11].
Electrolytes can be described as charged minerals ions that are found in living organisms [12]. These electrolytes include sodium, potassium, chloride, calcium and bicarbonates. Sodium and potassium are the major cations of the extracellular fluids while chloride and bicarbonates are the major anions of the intracellular fluids.[13]. They are grouped as divalent and monovalent ions according to functions and ionic capacity, the divalent ions (Calcium and Magnesium) are important in neuromuscular excitability, enzymatic reactions and retention of membrane permeability[1,4] Electrolytes are needed for osmo-regulatory purposes in the body system of living organisms, thus electrolyte balance in the body of organism is necessary for the normal function of cells and organs. Gabriel et al. [11], revealed that the basic function of electrolytes in the body include the control of fluid distribution, intracellular and extracellular acido-basic equilibrium so as to achieve proper maintenance of osmotic pressure of body fluids and normal neuro-muscular irritability. Therefore, alterations of the electrolyte balance of an organism would adversely affect the organism concerned [14]. In fish inhabiting a freshwater, blood ionic concentrations are maintained at much higher levels than those of the ambient water. Hence, they constantly face osmotic in-flow of water and diffusion losses of ions across the body surface and gill epithelium. A disturbed hydro- mineral balance of the body fluids of fish is one of the most conspicuous phenomena observed during stress as there exists an intimate relationship between the surrounding water and the body fluids [15]. This paper therefore assessed the effects of different chemicals on the electrolyte profiles of H.longifilis, a commonly culture fresh water fish in Nigeria.
Materials and Methods:
Experimental Location and Fish:
The study was carried out in Aqua Green Integrated Aquaculture Center, Port Harcourt, Rivers State, Nigeria. One hundred and thirty five H.longifilis juveniles (mean length 22.71±5.91 cm; mean weight 300.19±12.99g)were sourced from a commercial farm in the State..
Preparation of Test Solutions and Exposure of Fish:
Three pesticides which include; paraquat dichloride (PARAQ), 2,2-dichlorovinyl phosphate (DDVP) and dimethoate (DMC) used in this experiment were purchased from a commercial outlet in Port Harcourt, Nigeria. The fish were exposed to each of the chemical at the concentrations of 0.00 control, 0.05, 0.10, 0.15, 0.20, and 0.25 mg/L in triplicates based on concentration from previous studies [6,11,12]. Five fish were randomly distributed into each test tank. The experiment lasted for a period of 15 days. The water in the tanks was renewed daily. The fish were fed twice daily at 3% body weight with a commercial feed.
Determination of blood serum electrolytes:
At the end of each experimental period, 2ml of fresh blood sample was collected by making a caudal puncture with the help of fine needle and poured in heparinized sample bottles. These bottles contain sodium heparin used for the collection of heparinized plasma or whole blood for biochemical tests [16]. Plasma was separated by centrifugation at 10,000rpm for 5-8 minutes in TG20-WS Tabletop High Speed Laboratory Centrifuge. Plasma electrolytes such as Na+, K+, Ca2+ and Cl- were determined by using Hitachi 902automatic analyzer (Japan), following the method described by Gabriel et al. (2010). All the tests were performed in triplicates.
Statistical Analysis:
The data obtained from this study was collated and analyzed using statistics software SPSS version 22. A two-way analysis of variance (ANOVA) was employed to reveal significant differences in measured variables among control and experimental groups. When a different was detected (P<0.05), Tukey’s (honestly significant difference) test was applied to separate which treatment was significantly different.
Results:
The resultant effects of paraquat dichloride on the electrolytes in the plasma of H.longifilis are presented in Table 1. It was observed that sodium ion (Na+) and K+ increased with increasing concentrations of the pesticides. Also, Ca2+ decreased significantly when compared to the control values. While the chloride values were within the same range of 23.0- 28.0 (Table 1). In the plasma of fish exposed to DDVP, these ions Na+, K+, and HCO3- fluctuated significantly (P<0.05) when compared to the control values. While Na+ and K+ increased and peaked at 0.25 mg/L of the chemical. The values of calcium ions reduced significantly with increasing concentrations of the chemical. Whereas chloride ions were within the same range of 23.00-26.00 across the concentrations in the exposed fish (Table 2). In the fish treated with DMC there was significant variation across the concentrations of exposure in all the ions. At the same time Na+ and K+ increased and peaked at 0.25mg/L of the chemical, also, the values of calcium ions reduced from 42.50±6.01 in the control fish to 25.17±6.01in the highest concentration of the chemical (Table 3). Comparatively, the values of Na+ in the plasma of H.longifilis exposed to different chemicals are presented in Figure 1. The values of Sodium increased with increasing concentrations of the chemicals. However, the fish exposed to Paraquat consistently recorded the highest values of Sodium ions in all concentrations; this was closely followed by DDVP, while those in Dimethoate recorded the lowest. Conversely, reverse was the case in the values of calcium ions, in H.longifilis exposed to different chemicals (Figure 2). The values of calcium ions decreased as the concentrations of the chemicals increased. Also, the lowest reduction was equally observed in the fish exposed to the chemical Paraquat (Figure 2). Moreover, the values of K+ in the plasma of H.longifilis exposed to different chemicals are presented in Figure 3. The values of Potassium ions increased with increasing concentrations of the chemicals. However, the fish exposed to Paraquat consistently recorded the highest values of Potassium ions in all concentrations; this was closely followed by DDVP, while those in Dimethoate recorded the lowest. While the values of chloride ions in the exposed fish had no definite trend (Figure 4).
|
|
Conc.(mg/L) |
Na+ |
Ca2+ |
K+ |
|
Cl- |
|||||
|
0.00 |
68.06 ±19.77a |
42.88 ± 7.02d |
12.40 ± 2.99a |
23.00 ± 2.02a |
|
||||||
|
0.05 |
74.50 ± 12.02a |
35.02 ± 2.55c |
13.80 ± 1.03a |
24.00 ± 3.04a |
|
||||||
|
0.10 |
80.80 ± 17.91b |
30.77 ± 7.45c |
15.50 ± 2.02a |
25.00 ± 2.71a |
|
||||||
|
0.15 |
83.90 ± 12.66c |
23.33 ± 6.11b |
17.10 ± 2.01b |
26.00 ± 2.59a |
|
||||||
|
0.20 |
95.50 ± 11.50c |
17.50 ± 1.04a |
20.03 ± 1.04c |
25.00 ± 3.43a |
|
||||||
|
0.25 |
118.99 ± 12.01d |
14.56 ± 3.11a |
24.20 ± 2.18c |
28.00 ± 3.77a |
|
||||||
Means in the same column with different superscripts are significantly different (p<0.05).
Table 1: Effects of Paraquat Dichloride on the electrolytes ions in the Plasma of H.longifilis (Mean±SD).
|
|
Conc.(mg/L) |
Na+ |
Ca2+ |
K+ |
Cl- |
||||||
|
0.00 |
67.99 ± 6.06a |
42.59 ± 6.01c |
12.42 ± 8.01a |
23.00 ± 2.17a |
|
||||||
|
0.05 |
69.20 ± 5.71a |
39.87 ± 7.55b |
12.40 ± 3.82a |
26.00 ± 7.03a |
|
||||||
|
0.10 |
71.10 ± 4.38b |
35.43 ± 8.21b |
14.40 ± 5.07a |
25.00 ± 5.03a |
|
||||||
|
0.15 |
78.90 ± 5.87b |
29.20 ± 9.11a |
16.60 ± 7.81a |
24.00 ± 6.43a |
|
||||||
|
0.20 |
89.70 ± 6.64c |
24.50 ± 5.33a |
18.80 ± 6.91b |
25.00 ± 5.11a |
|
||||||
|
0.25 |
100.44 ± 7.22d |
20.99 ± 7.01a |
20.90 ± 5.02b |
26.00 ± 4.88a |
|
||||||
Means in the same column with different superscripts are significantly different (p<0.05).
Table 2: Effects of 2,2-dichlorovinyl phosphate on the electrolytes in the Plasma of H.longifilis (Mean±SD).
|
|
Conc.(mg/L) |
Na+ |
Ca2+ |
K+ |
Cl- |
||||||
|
0.00 |
68.02 ± 6.01a |
42.50 ± 6.01c |
12.40 ± 4.18a |
23.00 ± 3.07a |
|
||||||
|
0.05 |
64.79 ± 7.11a |
33.11 ± 7.44b |
12.58 ± 4.01a |
24.00 ± 5.91a |
|
||||||
|
0.10 |
66.21 ± 9.13a |
30.99 ± 5.09b |
13.08 ± 5.06a |
24.00 ± 4.03a |
|
||||||
|
0.15 |
70.12 ± 8.66b |
28.88 ± 9.314a |
14.39 ± 3.01a |
25.00 ± 3.02a |
|
||||||
|
0.20 |
84.65 ± 7.51b |
27.86 ± 8.13a |
16.19 ± 3.88b |
24.00 ± 2.29a |
|
||||||
|
0.25 |
96.01 ± 9.17c |
25.17 ± 6.01a |
18.99 ± 8.07b |
25.00 ± 3.67a |
|
||||||
Means in the same column with different superscripts are significantly different (p<0.05).
Table 3: Effects of Dimethoate (DMC) on the Electrolytes ions in the Plasma of H.longifilis (Mean±SD)
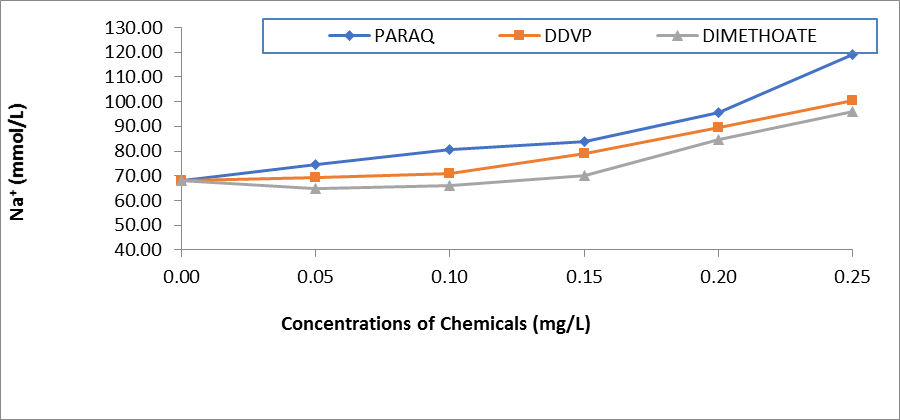
Figure 1: Comparative Values of Sodium ions (Na+) in the Plasma of H.longifilis Exposd to Different Chemicals.
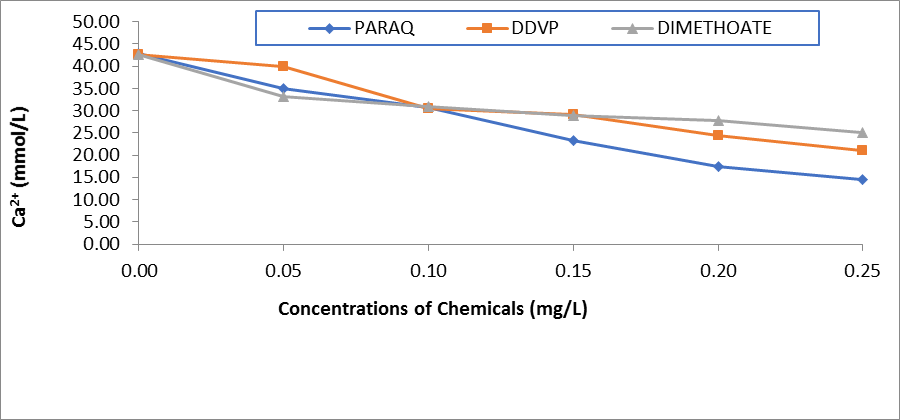
Figure 2: Comparative Values of Calcium ions (Ca2+) in the plasma of H.longifilis Exposed to Different Chemicals
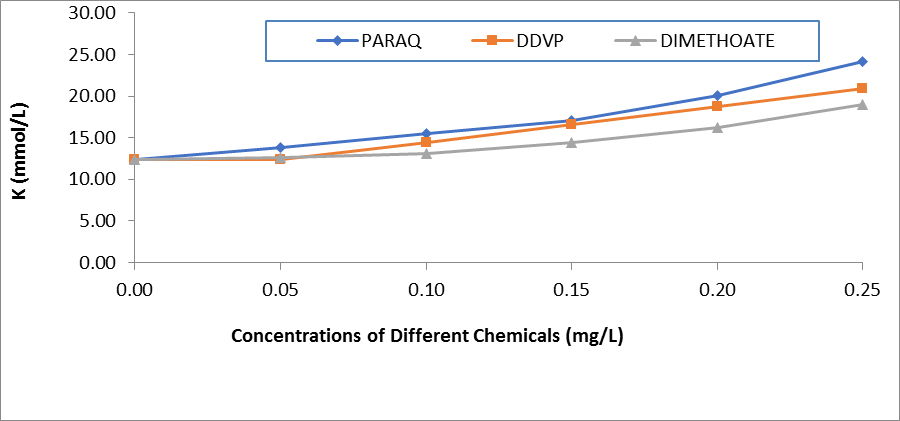
Figure 3: Comparative Values of Pottassium ions (K+) in the plasma of H.longifilis Exposed to Different Chemicals
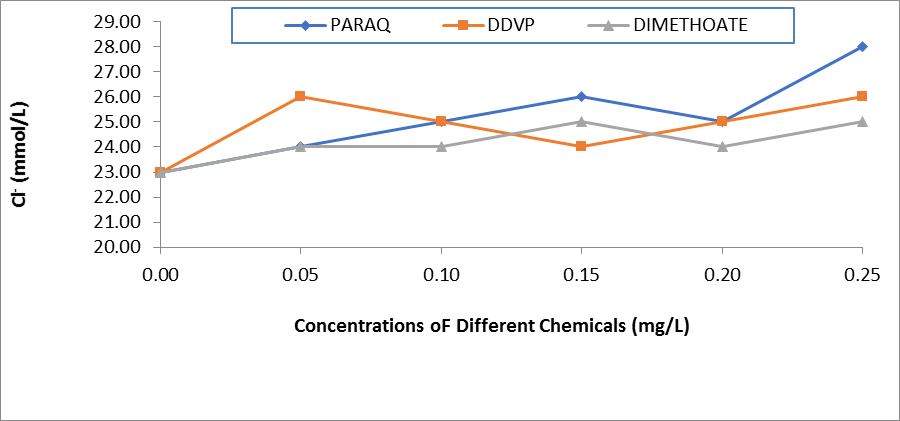
Figure 4: Comparative Values of Chloride ions (Cl-) in the Plasma of H.longifilis exposed to Different Chemicals
Discussion:
In an attempt to define and measure the effects of pollutants on aquatic organisms, biomakers and bioindicators are employed in several studies on xenobiotics. The principle of this approach is the analysis of electrolytes [17]. As Gabriel et al. [6] opined that toxicants act by the disruption of cell membrane permeability replacing the structural or electro-chemical important element in the cell which causes functional failure. Moreover, the basic function of electrolytes in the body lies in controlling fluid distribution, inter and extra cellular acids, basic equilibrium, maintaining osmotic pressure of body fluids and normal neuro muscular irritability 14]. Electrolyte concentrations have been used as an indicator of fishes’ ability to osmo-regulate [11]. This is often compromised with stress, disease or gill lesions that increase gill permeability to ions or lateral line imbalance and hormonal disorder [12].
Changes in the chloride electrolytes level in this study had no trend in all the plasma of the fish studied. The results is agreement with the report of Nte and Akinrotimi [13], who observed the same trend in chloride ions of S.melanotheron exposed to sub lethal levels of industrial effluents. This results could be based on the methods of analysis used and various factors both exogenous and endogenous.[18].As fish disturbed osmotic environment behaviorally adjust their electrolytes [19].This further supports the lack of trend in the results of chloride in the present study. The lack of trend in the chloride electrolytes analyzed in the plasma of H.longifilis exposed to different chemicals may be due to stress induced degeneration of the blood tissue [20]. Alterations in the values of chlorides as observed in this study may be due to induced stress from the toxicant. The balance of chloride ion is closely regulated by the body. Alterations in chloride ion when compared to the control value, may be due to certain kidney disease or over reactivity of the parathyroid glands and can also arise from gill injury due to the effect of the toxicant [21].
Electrolytes such as Na+, K+ and Ca2+ are the major cations of the extra cellular fluid. Their concentrations in the blood are indicative of a fish’s ability to osmoregulate properly [22]. The higher values of blood electrolytes such as sodium and potassium ions and lower values of calcium ions in the fish exposed to different chemicals in this study may be an indication of gill and kidney damage, which may have affected the osmoregulatory ability of the exposed fish. Change in pathology of the fish can also be due to electrolyte imbalance which in turn affects the fish physiology [23]. This results is in tandem with that of Inyang and Patani [24] who observed same in H.bidorsalis exposed to chemical Rhonasate in the laboratory. Also, the same trend was however observed in C.gariepinus exposed to cypernethrin [25] According to Bansal et al.[26], these minerals or electrolytes have the major responsibility of maintaining osmotic pressure in the blood and proper function of all types of tissues. Their imbalance due to the presence of toxicants may lead to the ability of the fish not to osmoregulate properly. The alterations in the levels of sodium, potassium and calcium ions in the plasma compared to the control may be attributed to kidney dysfunction, the kidney being the normal passage for these minerals [27].
Conclusion:
In conclusion, the increased electrolytic activities in this study may be correlated with cell membrane damage or changed permeability caused by the chemicals. While, the decreased profile of these electrolyte estimated in this study is attributed to adverse effect of chemicals on cell and its organelles. Since all electrolytes are basically membrane bound and hence any perturbation in membrane property as a result of interaction with surfactants could lead to alteration in its activities, this study has shown that different chemicals induced different levels of severe metabolic crisis in the exposed fish. Hence, these chemicals should be prevented from gaining access to the aquatic environment.