
Laid Ziane1, 2, Abdelaziz Berreghioua1, 2, Mohammed Djellouli2
1Laboratory of Chemistry and Science Environment, Tahri Mohammed Bechar University. BP 417 Bechar 08000, Algeria.
2Department of Material Sciences, Faculty of Exact Sciences, Tahri Mohammed Bechar University. BP 417 Bechar 08000 Algeria.
*Corresponding Author: Abdelaziz Berreghioua, Laboratory of Chemistry and Science Environment, Tahri Mohammed Bechar University. BP 417 Bechar 08000, Algeria.
Received Date : November 24, 2022
Accepted Date : January 24, 2023
Published Date : February 22, 2023
Citation: Laid Ziane, Abdelaziz Berreghioua, Mohammed Djellouli. (2023) “ Chemical characterisation of the flavonol glycosides constituents, Total phenolic and flavonoid quantification of bioactive extracts from the leaves of Atriplex halimus.”, Aditum Journal of Clinical and Biomedical Research, 6(2); DOI: http;//doi.org/02.2023/1.1098.
Copyright: © 2023. Abdelaziz Berreghioua Emeka. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
In the current investigation, total phenolic and flavonoid contents in butanolic extract were found to be 68.20 mg gallic acid equivalent (GAE)/g dry extract) and 439 mg quercetin equivalents (QE)/g dry extract.
From the leaves parts of Atriplix halimus, two flavonol glycosides, named rhamnetin-3-O- glucopyranoside and rhamnocitrin-3-O-glucopyranoside were isolated by various chromatography methods. The structure elucidation of these compounds was established by various spectral techniques such as IR, NMR, mass spectroscopy and compared with literatures.
atriplix halimus; chromatography; flavonol glycoside; structural identification
Introduction:
Ensiling A. halimus as a browse forage showed similar outcomes to PEG supplementation. Within the mean time this system is probably less difficult and might decrease feeding cost than daily PEG supplementation. Consequently, it is probably greatest than the later one in developing nations to ameliorate the anti-nutritional elements effect of browsed plant species on ruminant farm animals (Abd El-Rahman et al., 2006).
The salt that penetrates the plant gathers in bladder cells on the leaf surfaces of a triplex halimus. The salt is then expelled as a result of the cells bursting (Wong et al., 1978 ). A. halimus plant life accumulates large amounts of Cd in their tissues (predominance in roots), implying that they could be used to decontaminate saline soils contaminated by Cd (Bouzid et al., 2009).
Medicinal plants used in traditional medicinal drugs are one of the most intriguing fields for the development of new tablets for the treatment of various diseases because to their therapeutic qualities (Djellouli et al., 2015).
The benefits of phytochemicals extracted from flora and their impact on human health were the focus of these studies. Compounds, businesses of com- pounds, and essential oils are examples of natural additions derived from flowers. Polyphenols have a wide range of biological effects, including antibacterial, anti-inflammatory, antiallergic, hepatoprotective, antithrombotic, and antiviral properties (Bey-Ould Si et al., 2016).
The chemical composition of A. halimus reveals the presence of secondary metabolites such as tannins, flavonoids, saponins, alkaloids, and resins, as well as up to 10% sodium chloride. It has a high ash and crude fiber content, a moderate crude protein concentration, and a low crude fat level (Benhammou et al., 2009).
This present paper deals with the isolation and structure determination of twonew flavonol glycosides (1–2) (Fig. 1), from the ethyl acetate fraction of the MeOH extract of the of this plant.
Materials and Methods:
General experimental procedure:
NMR spectra were recorded in CD3OD using a Bruker GP-400 (300 MHz for 1H-NMR and 100 MHz for 13C-NMR) spectrometer. The high resolution electrospray ionisation mass spectroscopy (HR-ESI-MS) was recorded on a Bruker MicroTOF-QII spectrometer (Bruker Daltonik GmbH, Bremen, Germany). UV spectra were obtained in MeOH solvent with UNICAM UV300 spectrophotometer. IR spectra were obtained with an AVATAR 320 FT-IR spectrophotometer. TLC was carried out on silica gel 60 F254 plates (Merck, Germany). For Column chromatography was performed over silica gel 60 (Merck, particle size 230-400 mesh).
Plant materials and chemicals:
Aerial parts of Atriplix halimus were collected in march 2016 from boukais (South Western Algeria) Algeria, The plants were identified and a voucher specimen was deposited at the herbarium of the Valorization of Resource and Food Security in Semiarid Areas Laboratory, South West of Algeria, University of Béchar (Maire et al., 1953 ; Schauenberg et al., 1977).
Extraction and isolation:
Air-dried and powdered leaves parts of Atriplix halimus (100 g) were exhaustively extracted with 80% MeOH (400 ml) at reflux. The combined extracts were concentrated under reduced pressure to dryness. The residue was suspended in H2O and partitioned with petroleum ether, EtOAc, and n-BuOH, successively.
The butanolic extract was subjected to silica gel column chromatography and eluted with gradient solvent system of chloroform – methanol (95–5) to afford six fractions: F1 (0.25 g), F2 (0.70 g), F3 (0.46 g), F4 (0.35 g), F5 (0.30 g), and F6 (0.42 g). Fraction F2 (0.7 g) was chromatographed on silica gel and eluted with CHCl3-MeOH (9.5:0.5) to obtain compounds 1 (52 mg). Fraction F3 (0.46 g) was separated by silica gel chro-matographic column using CHCl3-MeOH (8:2), and further separated by RP-18 using gradient mixtures of CHCl3-MeOH (2:1) to affrord compound 2 (40 mg) (Hamidi et al., 2012 ; Ziane et al., 2015 ; Phana et al., 2016).
Total phenolic quantification:
Standard process designed the procedure. For the quantification of total polyphenols, this method has been used. Each sample extract was transferred to a 25 mL volumetric flask containing 2.5 mL of 3.54 g.L-1 Iron(III) chloridehexahydrate (FeCl3.6H2O) solution. The sample solution was then placed in a volumetric flask and kept at 80°C in a water bath for 20 min. Following that, 2.5 mL of acetate buffer (CH3COOH/CH3COOK) solution (pH 4.6), 5.0 mL of 3.28 g.L-1 1,10-phenanthrolinehydrate (1,10-phen), and 2.5 mL of 3.72 g.L-1 Ethylene diaminetetraaceticaciddihydrate (EDTA) solutions were added, in that order. Finally, each flask was filled with distilled water to the specified level, chilled, and absorbance measurements were taken at 511 nm (Kumar et al., 2017).
Total flavonoid quantification:
The total flavonoid content of the plant extracts was determined by producing different aliquots of the extracts. 0.1 mL 10 percent aluminum chloride and 0.1 mL potassium acetate (1 M) were added to this method, and the final volume was increased to 3 mL by adding distilled water. The samples were then incubated at room temperature for 30 minutes.
The calibration curve was created by reading the absorbance at 415 nm and using quercetin as a reference. The total flavonoid content was quantified using the standard curve of quercetin and the results were represented in milligrams of quercetin equivalents (QE) per gram of dry extract (mg QE/g of dry extract) (Rajappa et al., 2018).
Results:
Using the Folin- Ciocalteu technique, the total phelolic content of all examined extracts was determined. The butanolic extract was shown to be the most active, with a total concentration of 68.20 ± 0.03 GAE mg/g in dry extract. However, ethyl acetate had 38.80 ± 0.11 mg GAE/g, but diethyl ether extract contained 26.40 ± 4.73 GAE mg/g, dry extract (Table 1).
The total flavonoid content of butanolic extract was 439 ± 2.77 mg QE/g of dry extract, indicating the presence of the most polyphenols in Atriplix halimus, followed by ethyl acetate extract with 411 ± 5.69 mg QE/g of dry extract (Table 1).
The methanolic extract of the leaves parts of Atriplix halimus was partitioned sequentially with Et2O, EtOAc and BuOH. The butanolic fraction was separated by combination of chromatographic methods to provide two flavonol glycosides. Rhamnetin-3-O- glucopyranoside and Rhamnocitrin-3-O-glucopyranoside (Phana et al., 2016; Ortega et al., 2017).
|
Extraction Solvents |
Total polyphenol content (mg GAE/g, dry extract) |
Flavanoid content (mg QE/g dry extract) |
|
Ethyl ether Ethyl acetate Butanolic |
26.40± 4.73 38.80± 0.11 68.20± 0.03 |
212 ± 4.15 411 ±5.69 439± 2.77 |
Table 1: Total phenolic and flavonoid contents (mg/g) of the Atriplix halimus
|
Position 1 2 |
|
Aglycone
6 6.22 (1H, d, J=2.1 Hz) 6.31 (1H, d, J=2.1 Hz) 8 6.49 (1H, d, J=2.1 Hz) 6.59 (1H, d, J=2.1 Hz) 2’ 7.52 (1H, d, J=2.0Hz) 8.09 (1H, d, J=8.5 Hz) 3’ 6.89 (1H, d, J=8.5 Hz) 5’ 6.72 (1H, d, J=9.1 Hz) 6.89 (1H, d, J=8.5 Hz) 6’ 7.82 (1H, d, J=9.1 Hz) 8.09 (1H, d, J=8.5 Hz) 7-OCH3 3.77 (3H, s) 3.89 (1H, s)
Glc
1’ 5.50 (1H, d, J=7.5 Hz) 5.47 (1H, d, J=7.1 Hz) 2’ 3.55 (1H, d, J=7.5 Hz) 3.59 (1H, d, J=9.2 Hz) 3’ 3.44 (1H, d, J=9.1 Hz) 3.54 (1H, d, J=9.2 Hz) 4’ 3.17 (1H, d, J=9.1 Hz) 3.25 (1H, d, J=9.2 Hz) 5’ 3.25 (1H, m) 3.39 (1H, m) 6’ 3.72 (1H, dd, J=10.1, 1.8 Hz) 3.80 (1H, dd, J=12.1, 2.1 Hz) 3.30 (1H, dd, J=10.1, 6.4 Hz) 3.42 (1H, dd, J=12.1, 7.1Hz) |
Table 2: 1H (400 MHz) spectroscopic data of compounds 1 & 2 (in CD3OD)
|
Position 1 2 |
|
Aglycone 2 158.2 159.6 3 133.5 135.5 4 178.2 180.1 5 162.2 163.4 6 98.4 99.6 7 167.2 168.0 8 92.8 94.3 9 158.1 157.8 10 105.6 104.8 1’ 123.2 122.9 2’ 116.9 133.1 3’ 144.8 116.5 4’ 148.1 160.8 5’ 118.2 116.5 6’ 124.3 133.1 7-OCH3 58.2 55.9 Glc 1’’ 101.2 100.2 2’’ 81.4 79.5 3’’ 78.6 78.1 4’’ 70.9 71.6 5’’ 76.8 76.4 6’’ 69.5 68.2
|
Table 3: 13C NMR (100 MHz) spectroscopic data of compounds 1&2 (in CD3OD)
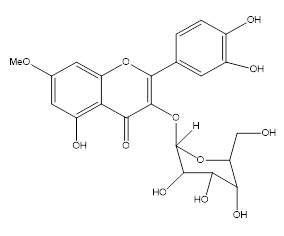
Figure 1: Rhamnetin-3-O- glucopyranoside (1).
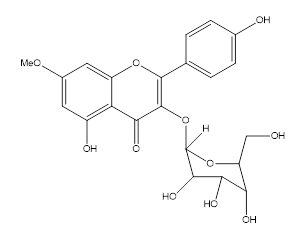
Figure 2: Rhamnocitrin-3-O-glucopyranoside (2)
Discussion:
Phenolics compounds were extracted by Soxhlet method and analyzed by the Folin–Ciocalteu colorimetric method, while flavonoids were determined by aluminum trichloride assay. All tested extracts contain phenolic compounds,however the most significant amount of total phenolic and flavonoid contents was presented in butanolic extract (68.20 mg GAE/g, dry extract and 439 mg QE/g, dry extract, respectively).
Rhamnetin-3-O- glucopyranoside (1), Fig (1) was isolated as a yellow amorphous powder. Tf= 159 °C, Rf = 0.46, UV λmax MeOH (nm): 350, 284, 266 (+NaOMe 269, 386, 372; +AlCl3 284, 321, 422; +AlCl3/HCl; 283, 319, 427; +NaOAc 284, 352,). IR (KBr): 3432, 2 951, 1 662, 1529, 1 485, 1331, 1 151, 1 034 cm-1. The molecular formula was determined to be C22H22O12. molecular ion peaks, m/z 478 [M+H]+.
The 1H NMR spectrum revealed the presence of a pair of meta-coupling aromatic ring at δH 6.22 and 6.49 (each d, J=2.1Hz), the aromatic protons at positions H-2 ', H-5' and H -6' which appear to the area without armor, with the chemical shifts: 7.52, 6.72, 7.82 ppm, respectively and a methoxyl singlet signal at δH 3.77 for the aglycone part in addition to anomeric proton for sugar moieties at δH 5.50 (1H, d, J=7.5 Hz). These results are confirmed by 13C NMR spectra, that presents 22 signals, six signals appears between 69.5-101.2 ppm corresponds to glucosid carbons, the more one signal appears to 178.2 ppm correspond to carbonyl group (C=O).
Rhamnocitrin-3-O-glucopyranoside (2), Fig (2) was isolated as a yellow amorphous powder. Tf= 165 °C, Rf = 0.54, UV λmax MeOH (nm): 346, 278, 262 (+NaOMe 279, 366; +AlCl3 285, 356, 410; +AlCl3/HCl; 288, 407; +NaOAc 280, 349). IR (KBr): 3335, 2 933, 1 654, 1512, 1 479, 1318, 1 143, 1 025 cm-1. The molecular formula was determined as C22H22O11. molecular ion peaks, m/z 462 [M+H]+. The 1H NMR spectrum (Table 2) showed a typical flavonoid pattern with a para-substituted B-ring characterized by two doublets at 8.09 ppm (H-2’’ and H-6’’, J = 8.5 Hz) and 6.89 ppm (H-3’’ and H-5’, J = 8.5 Hz), each integrating for two protons. A substituted A-ring carrying a methoxy group was evident from a singlet at 3.89 ppm, and two singlet at 6.31, 6.59 ppm indicated two proton for H-6 and H-8 respectively.
The 13C NMR spectrum displayed 22 carbon signals, including one carbonyl (δC 180.01), 14 aromatic carbons for three aromatic rings, one methoxy (δC 55.9), and six carbons for glucosid.
The chemical shifts indicated that compound 2 (Tables 2 and 3). As well as the sugar moiety as compound 1. Consequently, 2 was established to be glucopyranoside.
Acknowledgements:
The authors thank Mr. Henri Doucet (Universite´ de Rennes1, Campus de Beaulieu, France) for NMR and HR-ESI-MS measurement.