
Abishkauf jenish beautlin1, Ashok Govindaraj1, Durgadevi1, Gurubharath Ilangovan2, Divyalakshmi1
1Department of Cardiology, Chettinad Academy of Research and Education, Kelambakkam – 603103.
2Department of Radiology, Chettinad Academy of Research and Education, Kelambakkam – 603103.
*Corresponding Author: Abishkauf jenish beautlin. Department of Cardiology, Chettinad Academy of Research and Education, Kelambakkam – 603103.
Received Date: June 28, 2023
Accepted Date: July 28, 2023
Published Date: August 05, 2023
Citation: Abishkauf jenish beautlin, Ashok Govindaraj, Durgadevi, Gurubharath Ilangovan, Divyalakshmi. (2023) “Comparing hs-CRP and MPO biomarkers of subclinical atherosclerosis in metabolic syndrome patientse.”, Aditum Journal of Clinical and Biomedical Research, 6(4); DOI: http;//doi.org/08.2023/1.10110.
Copyright: © 2023. Abishkauf jenish beautlin. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Cardiovascular disorders have been linked to myeloperoxidase, an enzyme generated from leukocytes. MPO creates an antibacterial system and has been many correlations between MPO, hs-CRP and cardiovascular disease. The present study has aimed to assess MPO, hs-CRP and subclinical atherosclerosis in metabolic syndrome patients by using FMD and CIMT parameters.
Methods: 75 metabolic syndrome affected patients had defined as per the criteria of IDF (International Diabetes Federation) which had been in this study. In addition, ultrasound doppler had been employed for determining the carotid intima medial thickness for both right and left (left and right CIMT) and brachial artery FMD. By employing the Immuno assay MPO and hs-CRP ultrasensitive ELISA kit, the concentration of MPO and hs-CRP is measured.
Results: A positive link between MPO with CIMT right and left (r value of right CIMT=0.723, p <0.05 and left CIMT r=0.712, p<0.01), hs-CRP with CIMT right and left (r value of right CIMT=0.613, p<0.05and left CIMT r=0.64, p<0.01)and a significant inverse correlation between MPO with FMD (r= -0.319,p<0.05), hs-CRP with FMD (r= -0.304, p<0.01) in metabolic syndrome patients.
Conclusion: Serum MPO and hs-CRP is positively linked with subclinical atherosclerosis in metabolic syndrome patients. According to this finding, understanding the mechanism behind the risk factors for cardiovascular disease can be aided by the development of new molecular markers for metabolic syndrome and subclinical atherosclerosis.
flow mediated vasodilatation; carotid intima medial thickness; metabolic syndrome; HS-CRP, myeloperoxidase
Introduction:
Syndrome X or the Metabolic syndrome (MS) from the clinical standpoint represents the combination of various metabolic abnormalities that contribute increased cardiovascular disease [1]. In CVD related complication, atherosclerosis is regarded as a systematic, chronic and progressive condition observed with one of the prolonged asymptomatic phases [2]. Due to the macrophages activation and cytokines release by expanded adipocytes, pro-inflammatory state serves a pivotal role in the syndrome pathogenesis. Estimation of C-reactive protein level in serum can document this pro-inflammatory state [3,4]. From the point of fact, the criteria were initially proposed by WHO to define Metabolic Syndrome, which has been evolved over the course of years. Currently, Metabolic Syndrome criteria remain the basis of IDF (International Diabetes Federation) criteria, multiple clinical & laboratory parameters constellation. Since there is a dire need for predicting CVD related complications, the recent interest in the investigation on numerous biomarkers and their influence in the prediction of cardiovascular events could greatly facilitate in devising a suitable model that compares clinical variables and patient’s demographics. High sensitivity CRP levels need to be evaluated in metabolic syndrome. The level of HDL and obesity had been regarded out of all metabolic syndrome features. High sensitivity CRP levels had been thoroughly analysed in obesity patients.
Gradually, atherosclerosis has developed as one of the subclinical conditions over the course of life and they eventually become apparent as peripheral arterial disease clinically. The early stage atherosclerosis is referred as pre- or sub- clinical atherosclerosis, when it is within the vascular walls that is, something starts to get changed, but the cardiovascular disease isn’t evident clinically. Interest over the past decade has been gained by detecting the upcoming disease before the clinical manifestations at this stage [5]. Three subclinical atherosclerosis measures remain coronary artery calcification, ankle-branchial index & carotid intima-media thickness which can possibly be quantified as well as non-invasively detected.
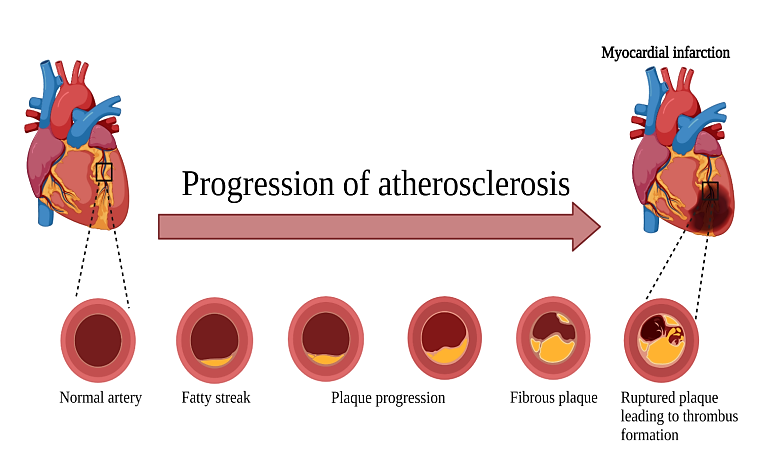
Figure 1: Progression of atherosclerosis
Materials and methods:
The investigation was done in chettinad Hospital and Research Institute, Kelambakkam. The period of study is extended between 2021 and 2022. The study population comprised of patients presented with metabolic syndrome were carefully selected on the basis of IDF (International Diabetes Federation) criteria. Based on the inclusion and exclusion criteria, every patient had been selected. High-sensitivity CRP and MPO had been estimated and analysed in all those patients. Both gender patients had been included in the study. About 75 patients had been included in this study. Predominantly, the sample had been collected from the patients who attended the master health check-up.
This investigation is a cross sectional study. Randomly, the study had been chosen from the master health examination patients. For metabolic syndrome, many quarters have approved and accepted the IDF guidelines. It has been the one that extensively employed in the clinical metabolic syndrome trials. 75 clinical samples are collected from metabolic syndrome patients.
The quantitative measurement was carried out using the enzyme-linked immunosorbent assay of MPO and hs-CRP in serum samples. Further, readings had been measured. As per the kit instructions, the procedure of ELISA had been performed.
Subclinical atherosclerosis parameters:
Experience radiologist took CIMT and FMD in the department of radiology.
Metabolic syndrome – there is the presence of more than or equal to three in the IDF criteria.
Raised triglyceride:>150 mg/dl
Reduced HDL cholesterol:<40 mg/dl in males and <50 mg/dl in females
Raised Blood Pressure: Systolic BP>130 or diastolic BP >85mm Hg
Raised fasting plasma glucose: FBG>100 mg/dL
Central obesity (Waist circumference) :(>80cm female) and (>90cm male)
Inclusion criteria:
The patient must meet the criteria for metabolic syndrome as outlined in the IDF recommendations previously mentioned.
Exclusion criteria:
FMD Analysis:
Assessment of brachial FMD was conducted among the study population in their fasting state. All the participants were instructed to lie down in a supine position for at least 10 minutes before the start of the test. A blood pressure cuff is placed around the participant's forearm, and an ultrasound probe is placed on the arm to visualize the brachial artery. The blood pressure cuff is inflated to a pressure of 200 mmHg to induce ischemia in the forearm. The cuff is then rapidly deflated, which leads to a reactive hyperaemic response that causes an increase in blood flow to the arm and a corresponding increase in brachial artery diameter. 70 to 90 seconds after the cuff deflates, the diameter of the brachial artery is measured. The FMD is calculated using the formula as follows: (maximum diameter – baseline diameter)/baseline diameter × 100%.
If the FMD was less than 10%, endothelial dysfunction was declared to be the cause.
CIMT:
CIMT (carotid intima-media thickness) is a safe procedure for assessing subclinical form of atherosclerosis. The methodology for CIMT measurement typically involves the following steps:
The participant is asked to lie down in a supine position, and the neck is slightly extended. An ultrasound probe with a high-frequency transducer (7-10 MHz) is placed on the skin over the carotid artery. The ultrasound probe is used to see the intimate-media complex of the carotid artery. The intima-media complex is the two innermost layers of the artery wall, which consist of the intima (the innermost layer) and the media (the middle layer). The CIMT is measured as the distance between the lumen-intima interface and the media-adventitia interface on both the left and right sides of the carotid artery. Measurements are taken at several points along the length of the carotid artery, typically at the common carotid, carotid bulb, and internal carotid segments. The mean CIMT is calculated as the average of the measurements taken at each segment of the carotid artery.
Statistical analysis:
SPSS IBM software version 26 software was used for data analysis. The mean and standard deviation (SD) were used to represent continuous variables. Correlation between the biomarkers were calculated by using Pearson’s correlation. The independent t-test was used to compare two independent populations . p value <0.05 was taken as statistical significance.
Results:
Study has 75 patients of which 47 were male (62.7%) and 28 were women (37.3%) with a mean age of 58.0 ± 10.2 years. Abnormal FBS ranges (N=69; 92%) with a mean average of 145.05 ± 50.720. In HDL (N=62;82.7%) patients were presented with abnormal with a mean average of 37.13 ±7.780. Abnormal ranges in TGL (N=50; 66.6%) with a mean average of 194.79±115.074. Abnormal ranges in waist circumference (N=23; 30.6%). Blood pressure in abnormal ranges(N=34;45.3%).
|
Parameters |
Mean ± Std. Deviation (Range) |
|
Age (years) |
58.08±10.247 (36-85) |
|
FBS in plasma (Normal 70–110 mg/dL) |
145.05±50.720 |
|
TGL (Normal < 150 mg/ dL) |
194.79±115.074 |
|
HDL (mg/ dL) |
37.13 ±7.780 |
|
Waist Circumference (inches) |
77.72 ±10.147 |
|
SBP (mm Hg) |
120.24±10.917 |
|
DBP (mm Hg) |
81.40±10.351 |
|
CIMT-Right (mm) |
1.0467±0.25697 |
|
CIMT-Left (mm) |
1.0160±0.31151 |
|
FMD (%) |
9.5240±2.514 |
|
hs-CRP (mg/ L) |
2.5467±1.328 |
|
MPO (AU/mL) |
26.66 ±6.96 |
|
|
|
Table 1: Baseline characteristics of the patients
|
MPO |
CIMT |
|
|
RIGHT
|
LEFT
|
|
|
26.66 ± 6.96 |
1.04±0.25** |
1.01±0.31** |
Table 2: Correlation of MPO with CIMT:
Considerably enhanced the diameter of the right and left CIMT as compared to the MPO with CIMT
Values expressed as mean standard deviation with pearson correlation significant levels of **p value <0.01(r value right CIMT=0.723 and left CIMT r=0.712)
|
Hs-CRP |
CIMT |
|
|
RIGHT
|
LEFT
|
|
|
2.54±1.32 |
1.04±0.25** |
1.01±0.31** |
Table 3: Correlation of hs-CRP with CIMT :
Compared to the HS-CRP with CIMT, the right and left CIMT's diameters are significantly increased.
Values expressed as mean standard deviation with pearson correlation significant levels of *p value <0.01 (r value of right CIMT=0.613 and left CIMT r=0.641)
|
MPO |
FMD |
|
26.6±6.96 |
9.52±2.51* |
Table 4: Correlation of MPO with FMD using Pearson Correlation :
Inverse correlation exists between the MPO and FMD.
Values expressed as mean standard deviation with pearson correlation significant values of *p value<0.05* (r= -0.319)
|
Hs-CRP |
FMD |
|
2.54±1.32 |
9.52±2.51* |
Table 4: Correlation of hs-CRP with FMD using Pearson Correlation
Inverse correlation exists between the hs-CRP and FMD.
Values expressed as mean standard deviation with pearson correlation inversely correlated (r= -0.304)
Discussion:
A measure of subclinical atherosclerosis called CIMT is reliable predictor of cardiovascular and cerebral vascular events. Carotid ultrasonography has been shown in studies to be a sensitive technique that produces trustworthy results [37]. Furthermore, it has been demonstrated that the onset of clinically discernible atherosclerotic plaques in the coronary arteries precedes, which is recognised as first event in atherogenesis. Nitric oxide (NO), in particular, has a lower bioavailability due to endothelial dysfunction, although the bioavailability of contracting substances originating from the endothelium is increased. Many studies evaluated the endothelial function as a predictor of CVD [38].
A high concentration of MPO is thought to be a predictor of future cardiovascular disease in healthy individuals since inflammation is activated many years before the beginning of cardiovascular disease. Even after adjusting for conventional risk variables and hypertension, increased the levels of MPO and hs-CRP were independently identified a predictor of endothelial dysfunction and cardiovascular disease [39].
According to recent research, hs-crp and serum MPO levels were predictive of subclinical atherosclerosis. As a result, increasing CIMT in MS patients was positively linked with higher levels of MPO and hs-CRP. According to Toprak and colleagues, who discovered that any rise in hs-CRP was connected to the advancement of CIMT regardless of other cardiovascular risk factors, there is a positive link between CIMT and hs-CRP (40). In the INVADE trial, which examined the relationship between MPO and CIMT development in aged patients, the two parameters were found to be independently correlated [41].
Our study demonstrates a substantial relationship between serum MPO and hs-CRP and alterations in FMD in brachial arteries. The consumption of nitric oxide, which may indicate a problem with endothelial function, is a significant effect of increased MPO activity. The endothelial dysfunction, was extensively evaluated in numerous research as a predictor of future cardiovascular events [42,43]. A loss of the unadjusted relationship between C-reactive protein and endothelial dysfunction after risk factor and cardiovascular disease adjustment. Due to these complicating factors, the previously established association between C-reactive protein and endothelial dysfunction may still hold true [44].
Our findings revealed favourable association between the biomarker ranges and subclinical atherosclerosis parameters in MS patients. This further suggest that the biomarkers could serve as a prognostic tool in detecting the complications and risks associated with CVDs that may contribute in the development of subclinical atherosclerosis. The study ultimately led us to the conclusion that the effects of hs-CRP and MPO could determine subclinical atherosclerosis condition. This showed that a simple blood test can be performed by any standard clinical laboratory and the versatility and possible clinical utility might be offered by MPO and hs-CRP testing in the disease advancement’s serial monitoring. It is further suggested that subclinical atherosclerosis and new biological markers for MS have been helpful in order to understanding the mechanisms and the role.
Conclusion:
Findings shows the serum biomarkers viz, MPO and hs-CRP were significantly correlated with subclinical atherosclerosis in patients with MS. It was indicated from the available data that hs-CRP selective determination has been beneficial in every individual with intermediate cardiovascular risks for optimizing the clinical management as well as risk stratification, in spite of limitations to the use of routine clinical practice, in particular, interindividual variability. In addition, on the basis of sound experimental evidences from the investigation linking with serum biomarkers with development of atherosclerosis represent a stronger association from the brachial FMD and CIMT measures observed in the current study, and earlier demonstration and promising as a biomarker target of subclinical atherosclerosis from the significant correlation in MPO and hs-CRP with subclinical atherosclerosis among metabolic syndrome affected patients. Cardiovascular risk stratification in metabolic syndrome affected patients with metabolic syndrome may be improved by the combination of ultrasonography alongside biomarker in association with vulnerable plaque pathophysiology namely MPO and hs-CRP. It is concluded that there is a strong relationship with regards to the elevated ranges in the biomarkers MPO and hs-CRP and the likely development in subclinical atherosclerosis condition among metabolic syndrome patients.