Aditum Journal of Clinical and Biomedical Research
OPEN ACCESS | Volume 8 - Issue 1 - 2026
ISSN No: 2993-9968 | Journal DOI: 10.61148/2993-9968/AJCBR
K.V. Ratnamala and Amena Amreen*
Rbvrr women’s college of pharmacy, Barkatpura, District Hyderabad, Telangana, India.
*Corresponding Author: Amena Amreen, Rbvrr women’s college of pharmacy, Barkatpura, District Hyderabad, Telangana, India.
Received Date: April 19, 2022
Accepted Date: May 06, 2022
Published Date: May 17, 2022
Citation: Amena Amreen and K.V. Ratnamala. (2022) “Niosome: Potential Carrier for Targeted Drug Delivery.”, Aditum Journal of Clinical and Biomedical Research, 4(3); DOI: http;//doi.org/05.2022/1.1079.
Copyright: © 2022. Amena Amreen. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Niosomes are a bilayered non-toxic, non-immunogenic nanoparticular delivery system that is widely used for drug delivery. Niosomes are gaining popularity as a coveted dug carrier due to their low cost and ease of manufacture. The main components of a niosome are cholesterol and a non-ionic surfactant, but several additives such as charged molecules (Solulan C24) can be added to modify the surface charge of the particle and improve its stability. Thin film hydration, micro fluidization, sonication, and the bubble method are just a few of the methods used to create niosomes. Because niosomes are amphiphillic molecules, they can entrap both hydrophilic and lipophilic drugs. There are numerous applications for niosomes in the pharmaceutical industry, the most important of which are cosmeceuticals, gene delivery carriers, vaccine delivery carriers, and medical imaging. This review attempted to document the key concepts relating to niosomes as well as the advances made in research in the field of niosomal drug delivery.
Introduction:
Niosomes are one of the most promising drug carriers in the nanoparticle industry. Niosomes have a bilayer structure and are formed by the self-association of nonionic surfactants and cholesterol in an aqueous phase. Handjanivila et al. discovered niosomes in 1979. Niosomes are microscopic lamellar structures formed by the admixture of a nonionic surfactant and cholesterol. They are formed by the self-assembly of amphiphilic molecules into closed bilayers. Because they have an amphiphilic bilayer structure, they can entrap both hydrophilic and hydrophobic drugs. As a result, niosomes are a novel and promising drug carrier [1].
Niosomes have a greater ability to penetrate than previous emulsion preparations. They have a bilayer and are structurally similar to liposomes; however, the materials used to prepare niosomes make them more stable, and thus niosomes offer many more advantages over liposomes. Because of their excellent skin penetration, niosomes have been the subject of extensive research into their potential as transdermal drug carriers. Niosomes are biodegradable, biocompatible, and nonimmunogenic. They have a long shelf life and are highly stable. Niosomes allow for controlled and/or sustained drug delivery at the target site [2].
Nonionic surfactants of various types have been reported to form niosomes, allowing the entrapment of a wide range of drugs with varying solubilities. To improve niosome drug delivery performance, the composition, size, number of lamellae, and surface charge of niosomes can be varied and optimised. The niosomal formulation of an anti-cancer drug reduces its systemic non-selective toxicity. Live demonstration of drug metabolism in areas where niosomes are present. The drug is also consumed by the liver and the enzyme lysosomal lipase.
It demonstrates that the niosome and the drug are degraded. entrapped in a niosome is released into the bloodstream, However, niosome degradation and breakdown in the liver take time. place very slowly as a result of which it shows more sustained effect [3]. Cholesterol plays an important role in the structure of the body. niosome, it gives vesicle rigidity, but when cholesterol is present, it loses its rigidity. When more quantity is added to a vesicle, it affects more than just the vesicle itself. The drug's fluidity, but also its penetration and permeability [4].
Definition of Niosomes:
Niosomes are synthetic microscopic vesicles consisting of an aqueous core enclosed in a bilayer consisting of an aqueous core enclosed in bilayer consisting of cholesterol and one or more non-ionic surfactants.
Structure and Composition of Niosomes:
The HLB value of the surfactant is primarily used in the selection of surfactant for niosome preparation. The ability of a surfactant to form vesicles is entirely dependent on its hydrophilic- lipophilic balance. The HLB value of the surfactant must be between 4 and 8 for proper and compatible niosome vesicle formation [5]. The niosome is a circular bilayer structure of nonionic surfactant, which must be able to form micelles. When the concentration of surfactant exceeds the critical micelle concentration (CMC), micelles form; however, non-ionic surfactants can form circular bilayer structures instead of micelles. Cholesterol is also added to the formulation to give the vesicle rigidity, and an ionic surfactant reduces aggregation [6]. Depending on the method used to prepare the niosome, the structure can be unilamellar or multi-lamellar. Hydrophilic, lipophilic, and amphiphilic drugs can all be incorporated into the structure of a niosome. The positions of all types of drugs in the niosome structure are depicted [7].
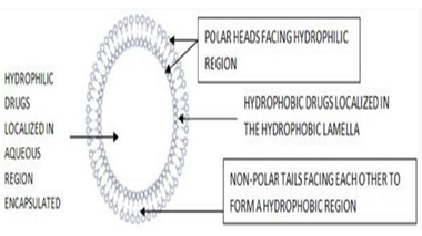
Figure 1: Niosomes structure
Salient Features of Niosomes:
Advantages of Niosomes:
Disadvantages of Niosmes:
Niosomes V/S Liposomes:
Niosomes and liposomes are functionally identical, with similar physicochemical properties depending on the bilayer composition and preparation methods. They both function as amphiphilic vesicles and can be used for both targeted and sustained drug delivery. Several authors have reported that niosomes function in vivo similarly to liposomes [11]. Niosomal and liposomal vesicular systems have similar applications in the pharmaceutical and cosmetic fields but differ chemically in their structure units; niosomes are made of surfactants, whereas liposomes are based on phospholipids, implying that niosomes are more stable and lack many of the properties of liposomes. The drawbacks associated with liposomes, namely their high cost, low availability, and the variable purity issues associated with phospholipids Niosomes do not require special conditions during preparation and storage, such as low temperatures or an inert atmosphere; these characteristics make niosomes more appealing for industrial manufacturing [12]. However, niosomes have several advantages over liposomes, including intrinsic skin penetration-enhancing properties [13].
|
|
NIOSOMES |
LIPOSOMES |
|
Component |
Surfactant |
Phospholipids |
|
Component availability |
High |
Low |
|
Component purity |
Good |
Variable |
|
Preparation and storage |
No special conditions required |
Inert atmosphere and low temperature |
|
Stability |
Very good |
Low |
|
cost |
Low |
High |
Table:1
Mechanism Action of Niosomes As Permeation Enhancer:
There is no single mechanism that can adequately explain niosomes' ability to increase drug transfer through the skin, and several mechanisms (Figure 3) have been proposed, including: alteration of the stratum corneum's barrier function as a result of reversible lipid organisation[14]; reduction of transepidermal water loss, which increases hydration of the stratum corneum and loosens its tightly- packed cellular structure[15]; and niosome adsorption and/or fusion on the skin's surface, as revealed by freeze fracture electron microscopy and small angle X-ray Scattering causes a high thermodynamic activity gradient of the drug at the interface, which is the driving force for drug permeation[16].
Adsorption of niosomes to the cell surface occurs with little or no internalisation of either aqueous or lipid components; it may occur as a result of attracting physical forces or as a result of specific receptors binding to ligands on the vesicle membrane and drug transfer directly from vesicles to the skin. On the other hand, niosomes can fuse with the cell membrane, causing the niosomal contents to be completely mixed with the cytoplasm. Finally, niosomes can be engulfed by the cell (endocytosis), with lysozymes in the cytoplasm degrading or digesting the niosomes membranous structure and releasing the entrapped material into the medium [17,18].

Figure 2: Mechanism of action of niosome as skin drug delivery system
Various Types of Niosomes:
Niosomes are classified into three groups based on vesicle size. Small unilamellar vesicles (SUV, size=0.025-0.05 m), multilamellar vesicles (MLV, size=0.05 m), and large unilamellar vesicles (LUV, size=0.10 m) are the three types.
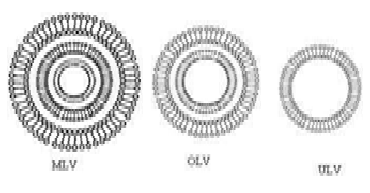
Figure 3: Different vesicle size of niosomes
Methods of Preparation:
Preparation of Small Unilamellar Vesicles:
Sonication:
The drug in the aqueous phase is mixed with the surfactant and cholesterol in a scintillation vial. For 3 minutes, a sonic probe is used to homogenise the mixture at 60°C. Small and uniform in size, the vesicles [19].
Micro Fluidisation:
Within the interaction chamber, two fluidised streams move forward through a precisely defined micro channel and interact at ultra-high velocities. A common gateway is set up in this case so that the energy supplied to the system stays within the area of niosome formation. As a result, there is greater uniformity, smaller size, and improved reproducibility [20].
Preparation of Multilamellar Vesicles:
Handshaking Method (Thin Film Hydration Technique):
In the hand shaking method, surfactant and cholesterol are dissolved in a rotary evaporator in a volatile organic solvent such as diethyl ether, chloroform, or methanol, leaving a thin layer of solid mixture deposited on the flask wall [21]. At room temperature and with gentle agitation, the dried layer is hydrated with an aqueous phase containing drug.
Preparation of Large Unilamellar Vesicles:
Reverse Phase Evaporation Technique (Rev):
In this method, cholesterol and surfactant are dissolved in an ether-chloroform mixture. A drug- containing aqueous phase is added to this, and the resulting two phases are sonicated at 4-5°C. After adding a small amount of phosphate buffered saline, the clear gel is sonicated again [22]. At 40°C and low pressure, the organic phase is removed. To produce niosomes, the viscous niosome suspension is diluted with phosphate-buffered saline and heated in a water bath at 60°C for 10 minutes.
Ether Injection Method:
The ether injection method is essentially based on slow injection of niosomal ingredients in ether through a 14-gauge needle at the rate of approximately 0.25 ml/min into a preheated aqueous phase maintained at 60°C. The probable reason behind the formation of larger unilamellar vesicles is that the slow vaporisation of solvent results in an ether gradient extending towards the interface of aqueous-nonaqueous interface. The former may be responsible for the formation of the bilayer structure [23]. The disadvantages of this method are that a small amount of ether is frequently present in the vesicles suspension and is difficult to remove.
Bubble Method:
It is a novel technique for preparing liposomes and niosomes in a single step without the use of organic solvents. It is made up of a round-bottomed flask with three necks that are placed in a water bath to control the temperature. The first and second necks contain a water-cooled reflux and thermometer, while the third neck contains a nitrogen supply [24].
At 70° C, cholesterol and surfactant are dispersed together in this buffer (pH 7.4). A continuous stream of nitrogen gas bubbles is generated and introduced through the dispersion, resulting in the formation of niosomes [25].
Formation of Niosomes From Proniosomes:
Another way to make niosomes is to coat a water-soluble carrier, such as sorbitol, with a surfactant. The coating process produces a dry formulation. Each water-soluble particle is coated with a thin layer of dry surfactant. This preparation is known as "Proniosomes." Niosomes are formed by adding aqueous phase at T > T m and agitating for a short period of time T = Temperature. Tm denotes the mean phase transition temperature.
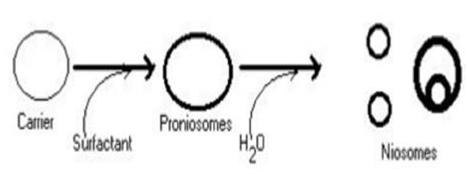
Figure 4: Niosomes formation
Separation of Entrapped Drug:
Various techniques, such as dialysis, gel filtration, and centrifugation, can be used to remove unentrapped solute from vesicles.
Evaluation:
Entrapment efficiency:
Following the preparation of the niosomal dispersion, the unentrapped drug is separated via dialysis centrifugation and gel filtration [28]. The drug is still entrapped in niosomes, as determined by complete vesicle disruption with 50 percent n-propanol or 0.1 percent Triton X-100 and analysis of the resultant solution using the following equation.
Entrapment efficiency= (Amount entrapped/total amount) *100
Size:
The shape of niosomal vesicles is assumed to be spherical, and the mean diameter of these vesicles can be determined using the laser light scattering method.
Electron microscopy, molecular sieve chromatography, ultracentrifugation, photon correlation microscopy, optical microscopy, and freeze fracture electron microscopy can also be used to determine the diameter of these vesicles [29].
Number of lamellae:
Nuclear Magnetic Resonance (NMR) spectroscopy, small angle X-ray scattering, and electron microscopy are used to determine this.
Membrane rigidity:
The mobility of a fluorescence probe as a function of temperature can be used to measure membrane rigidity [30].
In-vitro release:
Dialysis tubing is used in an in-vitro release rate study method. Distilled water is used to clean and soak a dialysis sac [31]. The vesicle suspension is pipetted and sealed into a bag made of tubing. The vesicles are placed in a bag with 200ml of buffer solution in a 250ml beaker with constant shaking at 25° C or 37° C. An appropriate assay method is used to analyse the buffer for drug content at various time intervals.
Microscopic evaluation:
The microscopic evaluation of niosomal dispersions was performed using transmission electron microscopy. TEM is used to determine the size of a sample and to determine whether it is spherical or not [31].
Applications of Niosomes:
For Controlled Release of Drugs:
However, niosomal vesicular systems have been proposed to improve drug bioavailability [32]. Multiple dosing with sodium stibogluconate loaded niosomes was found to be more effective against parasites in the liver, spleen, and bone marrow than a simple solution of sodium stibogluconate, according to Carter et al.
To improve the stability and physical properties of the drugs:
Delivery of peptide drugs:
Yoshida et al looked into the stability of peptides that had been made more stable by niosomes. Yoshida et al used an in-vitro intestinal loop model to deliver 9-desglycinamide, 8-arginine vasopressin entrapped in niosomes and found that niosomes increased peptide stability [33].
To increase oral bioavailability:
The oral bioavailability of acyclovir and griseofulvin was increased with the addition of niosomes to the drug when compared to the drug alone. Similarly, when administered as micellar solution along with the POE-24-cholesteryl ester, the absorptivity of poorly absorbed peptide and ergot alkaloid can be increased in the bile duct of rats.
neoplasia:
Doxorubicin, an anthracyclic antibiotic with broad-spectrum antitumor activity, has a dose-dependent antirreversible cardiotoxic effect. When administered via niosomal delivery to mice bearing S-180 tumours, this drug increased lifespan and decreased the rate of sarcoma proliferation [34].
use in studying immune response:
Because of their immunological selectivity, low risk, and higher solidity, niosomes are being used to investigate the concept of the immune reaction induced by antigens. Nonionic surfactant vesicles have clearly demonstrated their ability to function as an adjuvant after parenteral administration of various distinct antigens and peptides [35].
Niosome in gene delivery:
To evaluate transfection productivity in rodent retinas, a novel niosome detailing based on the 2,3-di (tetradecyloxy) propan-1-amine cationic lipid was combined with squalene and polysorbate 80.
Lipoplexes with a 15/1 ratio measured 200 nm, had a zeta potential of 25mV, and had a circular morphology [36]. At this concentration, niosomes consolidated and protected the DNA from enzymatic processing.
transdermal delivery of drug by niosomes:
In transdermal route of delivery, when drug is incorporated in niosomes penetration of drug through skin is enhanced [37].
For Targeting and Retention of Drug in Blood Circulation:
leishmaniasis:
Niosomes can be used for medication focusing in the treatment of diseases in which the contaminating life form lives in the organ of the reticulo-endothelial framework. Leishmaniasis is an infection in which the parasite attacks the liver and spleen cells [36].
Niosomes as carrier for haemoglobin:
Because niosomal suspension has a visible spectrum that is superimposed on that of free haemoglobin, it can be used as a haemoglobin carrier.
Vesicles are also permeable to oxygen, and the haemoglobin dissociation curve can be altered in the same way that non-encapsulated haemoglobin can [38].
Usefulness of niosomes in cosmetics:
|
Applications |
Components |
Method used |
Drug used |
|
As a drug delivery carrier |
Span 80, Cholesterol, Choloroform. |
Thin layer evaporation technique |
5-Fluorouracil (5-FU) |
|
To increase bioavailability |
Cholesterol, Sorbitan monostearate (span 60), Dicetylphosphate (dcp). |
Film hydration method |
Acyclovir |
|
For brain targeting |
N-palmitoyl glucosamine (NPG), Span 60 Cholesterol, Solulan C24. |
Thin film method, Ether injection method, Probe sonication method. |
Griseofulvin |
|
For drug targeting |
Palmitic acid, N- Hydroxy succinimide, Glucosamine, |
Sonication method. |
Transferrin. |
|
|
Span,60,40, DCP, Cholesterol. |
|
|
|
In anticancer therapy |
Cholesterol, DCP, Tween 20,60, Span 20,60,40, glycerol ether. |
Thin layer hydration method, sonication method. |
Doxorubicin |
|
In localized paralysis |
Chitosan phosphatidyl choline, Span 60, Cremophor RH40, Cholesterol, Butylated hydroxy toluene. |
Lipid layer hydration method. |
Methotrexate dithranol |
|
In oral delivery of peptide drug |
Brij 52,72,92,76,97,58,35, DCP, Cholesterol. |
Film hydration method |
Insulin |
Table:2
Conclusion:
In a nutshell, when compared to liposomes, niosomes are osmotically active and chemically stable on their own, as well as improving the stability of the drug entrapped and delivered. They do not necessitate special handling, protection, or storage conditions, nor do they necessitate industrial manufacturing. Aside from that, they provide structural flexibility (composition, fluidity, and size) and can be designed as desired.
Niosomes have several advantages over other drug delivery devices and have found use in the pharmaceutical industry.
It was thus concluded that niosomes are very effective drug delivery tools for the incorporation/targeting of various therapeutically active moieties, and the onus is now on future scientists to effectively harness its potential in diverse application areas for the benefit of humanity.