Aditum Journal of Clinical and Biomedical Research
OPEN ACCESS | Volume 8 - Issue 1 - 2026
ISSN No: 2993-9968 | Journal DOI: 10.61148/2993-9968/AJCBR
Ezeobi, A. J.1*, Pam, V. A.1, Uzoigwe, N. R.1, Omalu, I. C. J.2, Ombugadu, A.1, Ahmed, H. O.1, Ameh, S. F.3, Tanko, N. S.4, Adejoh, V. A.1, Attah, A. S.1, Ayim, J. O.1, Daramola, O. S.1, Aimankhu, P. O.1, Maikenti, J. I.1, Ajah, L. J.1, Ayuba, S. O.1, Aliyu, A. A.1, Ashigar, M. A.1, Odey, S. A.1, Anyebe, G. E.1, Kure, M. S.1
1Department of Zoology, Faculty of Science, Federal University of Lafia, P. M. B. 146, Lafia, Nasarawa State, Nigeria.
2Department of Animal Biology (Parasitology and Tropical Diseases Research Unit), Federal University of Technology, Minna, Niger State, Nigeria.
3Department of Toxicology and Pharmacology, National Institute of Pharmaceutical Research and Development, Federal Capital Territory, Abuja, Nigeria.
4Department of Chemistry, Faculty of Science, Federal University of Lafia, P. M. B. 146, Lafia, Nasarawa State, Nigeria.
*Corresponding Author: Ezeobi. A. J, Department of Zoology, Faculty of Science, Federal University of Lafia, P. M. B. 146, Lafia, Nasarawa State, Nigeria.
Received: December 27, 2021
Accepted: January 17, 2022
Published: January 31, 2022
Citation: Ezeobi. A. J, Pam. V. A, Uzoigwe. N. R, Omalu. I. C. J, Ombugadu. A, Ahmed. H. O, etc.al. (2022) “Anti-Trypanosomal Activity of Bufonidae (Toad) Venom Crude Extract on Trypanosoma brucei brucei in Swiss Mice.”, Aditum Journal of Clinical and Biomedical Research, 4(1); DOI: http;//doi.org/01.2022/1.1070.
Copyright: © 2022 Ezeobi. A. J. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Trypanosomiasis afflicts 6 – 7 million people globally and it is one of the major obstacles to livestock production in Africa. Naturally, trypanosomal parasites undergo genetic mutation and have developed resistance over a wide range of therapies. The utilization of animals and plants products have presented therapeutic potential for identifying novel anti-trypanosomal drugs. This study evaluated toad venom for anti-trypanosomal potency in-vivo in Swiss mice. Toads were collected from July to August 2019. The acute oral toxicity and biochemical characterization of the toad venom was determined. The experimental mice were administered various doses (130, 173 and 217 mg/kg) of the toad venom crude extract and 0.75mg/ml of Diamizan Plus standard drug for the treatment of trypanosomiasis, once daily for 3 days. The in-vivo anti-trypanosomal activity was evaluated by a curative test, after infecting the mice with Trypanosoma brucei brucei.The pre-patent period was 72 hours before treatment commenced. The overall results showed that trypanosomal load was highest in the control group while the group treated with Diamizan drug had the least trypanosomal load. As such, the mean trypanosomal load in relation to treatments showed a very high significant difference (P<0.05). Also, the mean trypanosomal load in Swiss mice in relation to the highest dosage of toad venom versus Diamizan drug showed a very high significant difference (P<0.05). The mean change in relation to the haematological parameters across treatments groups varied significantly (P<0.05) with the exception of Hb which showed no significant difference (P>0.05) across treatment groups. The over 50% reduction in the trypanosomal load in the 130mg/kg group in comparison with the control group brings to bare the anti-trypanosomal potency of the toad venom. The anti-trypanosomal activity demonstrated by the toad venom has provided basis for development of new therapeutic agents from different toad species. The study recommends further studies (both in-vivo and in-vitro) followed by the isolation and characterization of the active compounds present in the toad venom responsible for the anti-tyrpanosomal activity observed alongside the management and conservation of these species.
Introduction:
Trypanosomiasis is a parasitic disease caused by species of flagellate protozoa belonging to the genus Trypanosoma which inhabit the blood plasma and various body tissues and fluids. These parasites are found in many animals but seem to be pathogenic only for mammals, including man (Kasozi et al., 2021). Human African trypanosomiasis (HAT), or sleeping sickness, is a life-threatening disease caused by trypanosome parasites that are transmitted by tsetse flies. HAT is found only in sub-Saharan Africa and mostly affects poor rural populations. Two subspecies of Trypanosoma brucei cause disease: T. b. gambiense in West and Central Africa, and T. b. rhodesiense in East Africa. HAT transmission requires the interaction of humans, tsetse flies and parasite reservoirs (humans, and domestic and wild animals) (WHO, 2021).
African animal trypanosomiasis (AAT) is a parasitic disease that causes serious economic losses in livestock from anemia, loss of condition and effects on reproduction. Losses in cattle are especially prominent (Spickler, 2018). Trypanosomes infect a large number of wild fauna (antelope species, warthogs, elephants, hippopotamus, lions, hyenas, jackals, caracals, and wild ruminants etc.) (Obanda et al., 2011; Mbaya et al., 2013; Kasozi et al., 2021) and domestic ungulate species (cattle, sheep, goats, horses, pigs, camels and dogs) (Desquesnes et al., 2013). Infections in wildlife are influenced by species and habitat (Anderson et al., 2011). Trypanosome species commonly found in wildlife species include T. vivax, T. brucei brucei, T. congolense and T. evansi (Mbaya et al., 2013).
While both human and animal trypanosomiasis continue to be present as major human and animal public health constraints globally, although chemotherapy and chemoprophylaxis represent the mainstay for its control (Holmes et al. 2004; Giordani et al., 2016). Worryingly, the inherent potential of trypanosomal parasite to undergo genetic mutation has led to its ability to successfully develop resistance over a wide range of therapies.
Uptake of natural products from animals and plants presents explorable therapeutic potentials and earlier studies of toad venoms from different species and their chemical basis have demonstrated new perspectives for their pharmaceutical use including the development of new therapeutic agents (Qi et al., 2018). Over the years yet, a novel licensed compound is unlikely to be available (Kasozi et al., 2021); hence the rationale for this study.
Materials and Methods:
Toad Collection:
The toads used for this study were collected from the month of July to August 2019in a well ventilated container between 07:00am and 10:00am hours daily in the rice fields at Gandu, Lafia LGA, Nasarawa State and conveyed to the Laboratory unit of the Department of Zoology, Faculty of Science, Federal University of Lafia, Nasarawa State for extraction.
Extraction of Crude Venom Extract from Toad:
The process of extraction/secretion of Bufonidae was realized by massaging and pressing the parotoids macro-glandules and collected with a petridish (Gao et al., 2010). The collected secretion was lyophilized and stored in a freezer (-20°C) at the Federal University of Lafia, Lafia, Nasarawa State.
Biochemical Characterization:
Determination of the quantitative and qualitative chemical components of the venom was achieved by Mass Spectrometry using a Mass spectrophotometer at the Spectral Laboratory and Services, Tudun, Wada Kaduna South, Kaduna, Nigeria. The Shimadzu Fourier transform Infrared Spectrophotometer- FTIR 8400 S was used for the determination of the functional units present in the venom. Gas Chromatography Analysis of toad venom was done using gas chromatography (Perkinelmer 8500). The Scanning Electron Microscope energy dispersive X-ray spectroscopy (SEM-EDS) Phenom Prox, manufactured by phenom World Eindhoven (Netherlands) was used to carry out the morphology analysis (that is; analysis of the chemical elements present, in the toad venom).
Experimental Animals:
Thirty Laboratory swiss mice, weighing between 12 to 45g were purchased from National Veterinary Research Institute Vom, Jos, Plateau State. All animals were fed with formulated feeds and water was administered ad libitum. The caring and experimental use of the mice was in accordance with the National Institutes of Health Guidelines for Care of Laboratory Animals. The animals were acclimatized for 7 days prior to their randomization into the various experimental groups.
Coding and Weighing of Animals:
Different codes were given to every animal that was employed in this study using a permanent marker to create marks of identification on a particular part of the body that is head (HD), Bark (BK), Tail (TL), Right side (RS), Left Side (LS), Right ear (RE), Left Ear (LE) etcetera. The weight of each animal was taken using top animal precision balance.
Toxicity Study:
The median oral lethal dose of the toad venom was determined in mice using Lorke’s method 1983 (Enejide et al., 2013). This method has two phases.
Phase 1 require 9animals. The nine animals were divided into three groups of three animals each. Each animal was administered with different doses (10, 100 and 1000 mg/kg) of the toad venom and animals, placed under observation for 24hours to monitor their behavior as well as mortality.
Phase 2 involves the use of 3 animals, distributed into three groups of one animal each. The mice were intoxicated with different doses (250, 500 and 750 mg/kg) of the toad venom and observed for 24hours for behavior and mortality as well.
Then the LD50 is calculated by the formula:

D0 = Highest dose that gave no mortality,
D100 = Lowest dose that produced mortality.
Parasite Species and Standard Inoculation:
Parasite was obtained from National Veterinary Research Institute (NVRI) Vom, Plateau State, Nigeria. Parasites were maintained through serial blood passage in mice wherein the mice previously infected with Nigerian strain of Trypanosoma brucei brucei and with high parasitemia level served as the donor. Donor mouse was anaesthetized with chloroform and blood (1ml) was extracted through cardiac puncture using 1ml needle and syringe and made up to 20ml with normal saline. Blood samples were taken such that 0.2 ml injected subcutaneously into the experimental animals (Ndungu et al., 2019).
Determination of Parasites:
Blood samples were collected by bleeding the tail vein of the infected mice. Thin blood smears were made on clean glass microscope slides. The films were dried in air and then fixed in methanol and stained with 10% Giemsa solution (WHO, 2015). The stained film was then observed under the binocular compound microscope and viewed for parasitemia. The percentage parasitemia were determined by counting the number of parasites on four or five fields.
Preparation of Treatment Solution:
The treatment doses of the venom were calculated based on the lethal dose (LD50), which is 30% of the LD50 (Sims, 2021). Therefore, the study calculated 15%, 20% and 25% of the lethal dose as the curative doses, which gave 130 mg/kg, 173 mg/kg and 217 mg/kg respectively.
Curative Study:
The animals were acclimatized 7 days prior to their randomization into various groups infected with T. brucei-brucei and then divided into five groups of five mice per group. The presence of parasites was confirmed in the mice 72 hours after inoculation and was taken as day 0, thereafter, treatment commenced once daily for three days. Group 1, 2, and 3 were orally treated with 130 mg/kg, 173 mg/kg, and 217 mg/kg dose of crude toad venom extract respectively while group 4 received 0.75mg/ml of Diamizan Plus (treatment drug for trypanosomiasis) intradermally,whereas group 5 (infected and untreated) was regarded as the control. The parasitemia of experimental mice were established before treatment was administered (Osonwa et al., 2017).
Hematological Parameters:
Using the methods described by Cheesbrough (2004), the Packed Cell Volume (PCV), haemoglobin (Hb) and erythrocyte (RBC) counts were determined. These parameters were determined for each mouse before infection and after treatment. Blood samples were collected from the tail of each mouse with a heparinized capillary tube with one end sealed with plasticine.
Statistical Analysis:
Data obtained were analyzed using R Console software (Version 3.2.2). Shapiro-Wilk normality test was carried out to determine normality in the distribution of the data. Thereafter, Kruskal-Wallis rank sum test was used to compare the mean of pooled trypanosomal load in swiss mice in relation to toad venom treatments and standard drug. One-way analysis of variance was used to compare daily changes in the mean load of trypanosomes in swiss mice in relation to toad venom treatments and diamizan plus standard drug. Mean change in body weight as well as in haematological parameters were compared using Kruskal-Wallis rank sum test. Level of significance was set at P < 0.05. Wilcoxon rank sum test with Bonferroni correction was used as post-hoc test for multiple pairwise comparisons of means where there was a significant difference between the treatments in pooled trypanosomal load, change in weight and as well as haematological parameters. While Tukey’s Honest Significant Difference (Tukey HSD) post-hoc test was used for multiple comparisons of means in daily trypanosomal load changes between the treatments.
Results:
Determination of the Biochemical Compounds in the Toad Venom Crude Extract:
The results of the biochemical analysis of the toad venom crude extract showed that 9,12-Octadecadienoic acid (Z,Z) compound was the most dominant at peak 3 as shown in Figure 1 which had a rate of 38.615 at a proportion of 49.76% (Table 1) followed by n-Hexadecanoic acid (29.04%) then Octadecanoic acid (7.03%), Squalene (5.48%), 1-Hexadecyne (3.87%), Butyl 9,12-octadecadienoate (2.95%) while Hexadecanedioic acid was the least compound (1.87%).
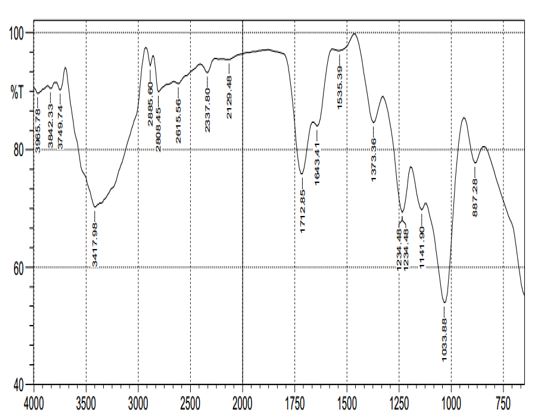
Figure 2 shows that the toad venom crude extract contains 18 active functional groups in which Nitrogen-Hydrogen Oxygen stretch of 93.18% was the most active functional group. The toad venom crude extract was predominantly characterized by the presence of the amino group made up of 8 functional groups, followed by the nitro-group having 6 functional groups while the non- protein group was made up of only 4 functional group as shown in Table 2.
The SEM-EDS analysis revealed that the toad venom crude extract contains 11 chemical elements, out of which carbon had the highest atomic concentration and weight of 61.76 mol/dm3 and 41.04 g/mol respectively, followed by oxygen (25.04 mol/dm3 and 22.29 g/mol) while iodine and aluminium had the least atomic concentration and weight of 0.24 mol/dm3 and 0.90g/mol respectively (Table 3).

Figure 1: Mass spectroscopy of toad venom crude extract showing the abundance of the chemical compounds at different peak
|
Peak |
Rate |
Library/ID of Compounds |
Percentage of Total Compounds (%) |
|
1
|
37.471 |
n-Hexadecanoic acid n-Hexadecanoic acid n-Hexadecanoic acid |
29.04 |
|
2
|
38.284
|
1-Hexadecyne 5-Hexadecyne 5-Eicosyne |
3.87 |
|
3
|
38.615 |
9,12-Octadecadienoic acid (Z,Z)- 9,12-Octadecadienoic acid (Z,Z)- 9,17-Octadecadienal, (Z) |
49.76 |
|
4
|
38.734
|
Octadecanoic acid Octadecanoic acid Pentadecanoic acid |
7.03 |
|
5
|
39.341 |
Hexadecanedioic acid Dodecanoyl chloride 4-Cyclopropylme thylbenzonitrile |
1.87 |
|
6
|
40.235
|
Butyl 9,12-octadecadienoate 6-Dodecane 7,10-Hexadecadienoic acid, methyl ester |
2.95 |
|
7
|
43.207
|
Squalene Squalene Supraene |
5.8 |
Table 1: The chemical components in the crude extract of the toad venom using the GC-MS
Frequency range (cm-1)
Figure 2: The functional groups present in the toad venom crude extract at different peaks
|
Amino-group |
Nitro-group |
Non-protein group |
|
Triethylamine
|
Nitro group with broad stretching of NO2 |
Aromatic group (Peak 4) |
|
Diethylamide
|
M-nitrotoluene |
Aromatic group (Peak 14) |
|
Aniline with a concentration of 84.653%
|
Nitrile group |
Carbon Hydrogen stretch with strong intensity of 89.60% |
|
Secondary Amines
|
Nitrogen Hydrogen Oxygen stretch of 93.18% |
Olefins with weak band of 53.975% |
|
n-butylamine & Benzamide with intensity (concentration) of 90.201%
|
Nitro Methane with concentration of 91.31% |
|
|
Nitrogen Hydrogen strong bond (amide) with concentration of 90.462
|
Nitrile group with Concentration of 89.86 % |
|
|
N-H bending |
|
|
Table 2: The different protein functional groups present in the toad venom crude extract at different peaks using FTIR
|
Element Number |
Element Symbol |
Element Name |
Atomic Conc. |
Weight Conc. |
|
6 |
C |
Carbon |
61.76 |
41.26
|
|
8 |
O |
Oxygen |
25.04 |
22.29
|
|
25 |
Mn |
Manganese |
4.12 |
12.59
|
|
82 |
Pb |
Lead |
0.94 |
10.84
|
|
24 |
Cr |
Chromium |
1.43 |
4.13
|
|
7 |
N |
Nitrogen |
2.67 |
2.08
|
|
53 |
I |
Iodine |
0.24 |
1.68
|
|
11 |
Na |
Sodium |
1.12 |
1.44
|
|
16 |
S |
Sulfur |
0.80 |
1.44
|
|
9 |
F |
Fluorine |
1.28 |
1.36
|
|
13 |
Al |
Aluminium |
0.60 |
0.90
|
Table 3: Chemical elements present in the toad venom crude extract using the SEM-EDS
Oral Toxicity (LD50) of the Toad Venom Crude Extract on the Laboratory Swiss Mice:
The oral toxicity test indicated that the toad crude venom extract resulted in mortality for 1000 mg/kg treatment after one hour. Prior to their mortality, hyperactivity, convulsion and constant stooling were observed before finally resulting in death. However, on the administration of 750 mg/kg, hyperactivity, diarrhea and sedation were observed within one hour, but there was no mortality recorded. Following the Lorke’s method computation, the oral toxicity of the toad crude venom extract was established as 866 mg/kg.
Trypanosomal Load at Day Zero:
Pre-patency was observed after 72 hours that was noted as day zero prior to the commencement of first treatment. Trypanosomal load was highest in mice group that were to be treated with 173mg/kg of the toad venom (group 2) followed by those designated in group 1 (130 mg/kg), then individuals for group 4 treatment(0.75 mg/ml of Diamizan plus), whereas parasitemia was very low in those set aside for group 3 (217 mg/kg) trial.However,prior to treatment, there was no significant difference (F20 = 0.9545, Adjusted R2 = -0.007644, P = 0.4537, Figure 3) in the mean of trypanosomal load in Swiss mice at day zero in relation to treatments with toad venom and Diamizan plus drug.

Figure 3: Mean trypanosomal load in Swiss mice in relation to treatments with toad venom and Diamizan plus drug at day zero
Trypanosomal Load in Swiss mice in Relation to Treatments with Toad Venom and Diamizan Plus Drug:
The overall trypanosomal load was highest in group 2 treated with 173mg/kg of toad venom crude extract followed by group 3 treated with 217 mg/kg then group 1 treated with 130 mg/kg while it was least in group 4 treated with Diamizan plus. Therefore, the mean trypanosomal load in Swiss mice in relation to treatments with toad venom and Diamizan plus drug showed a very high significant difference (Kruskal-Wallis c2 = 42.189, df = 4, P < 0.0001, Figure 4).Table 4 shows multiple comparisons between means of trypanosomal load in Swiss mice in which the highest dosage of toad venom versus Diamizan plus drug showed a very high significant difference (P < 0.0001). Also, trypanosomal load in Swiss mice between control group and the highest dosage of toad venom treatment was significant (P = 0.038).
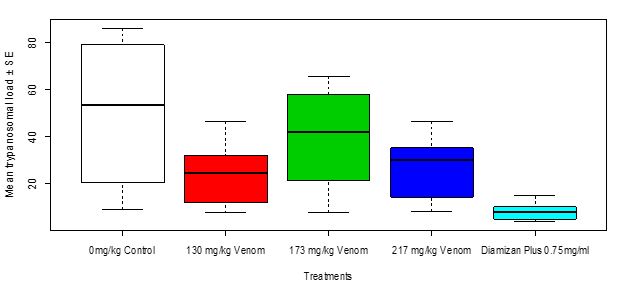
Figure 4: Mean Trypanosomal Load in Swiss mice in Relation to Treatment with Toad Venom and Diamizan Plus Drug
|
|
Trypanosomal Load and Treatments |
|||
|
|
0mg/Kg |
130mg/Kg |
173mg/Kg |
217mg/Kg |
|
130mg/Kg |
0.038 |
0 |
0 |
0 |
|
173mg/Kg |
0.156 |
0.038 |
0 |
0 |
|
217mg/Kg |
0.038 |
0.626 |
0.093 |
0 |
|
Diamizan Plus 0.75mg/ml |
1.0e-05 |
1.0e-05 |
1.0e-05 |
2.7e-05 |
Table 4: Pairwise Comparisons between Means of Pooled Trypanosomal Load in Swiss mice in Relation to Treatments with Toad Venom and Diamizan Plus Drug using Wilcoxon Rank Sum Test
P Value Adjustment Method: BH
Trypanosomal Load at Day One:
The trypanosomal load at day one (after 24 hours of treatment) was highest in group 2 (173 mg/kg of the toad venom), followed by group 1 (130 mg/kg of the toad venom), then group 3 (217 mg/kg of the toad venom), whereas it was least in group 4(0.75 mg/ml of Diamizan plus). Therefore, the mean trypanosomal load in Swiss mice in relation to treatments with toad venom and Diamizan plus after 24 hours showed a very high significant difference (F20 = 19.43, Adjusted R2 = 0.7544, P = 0.000001157, Figure 5). The comparison of trypanosomal load in Swiss mice in relation to the highest dosage of toad venom versus Diamizan plus treatments in day 1 showed a very high significant difference (P < 0.0001) as shown Table 5.

Figure 5: Mean trypanosomal load in Swiss mice in relation to treatments with toad venom and Diamizan plus treatments at day one
|
Treatment |
Diff |
Lower |
Upper |
P – Adjusted |
|
130mg/kg – 0mg/kg |
-9.75 |
-18.5138779 |
-0.9861221 |
0.0246790 |
|
173mg/kg – 0mg/kg |
-1.80 |
-10.5638779 |
6.9638779 |
0.9710613 |
|
217mg/kg – 0mg/kg |
-8.45 |
-17.2138779 |
0.3138779 |
0.0621310 |
|
Diamizan Plus 0.75mg/ml – 0mg/kg |
-23.15 |
-31.9138779 |
-14.3861221 |
0.0000013 |
|
173mg/kg – 130mg/kg |
7.95 |
-0.8138779 |
16.7138779 |
0.870134 |
|
217mg/kg – 130mg/kg |
1.30 |
-7.4638779 |
10.0638779 |
0.9913229 |
|
Diamizane Plus 0.75mg/ml – 130mg/kg |
-13.40 |
-22.1638779 |
-4.6361221 |
0.0015377 |
|
217mg/kg – 173mg/kg |
-6.65 |
-15.4138779 |
2.1138779 |
0.1954923 |
|
Diamizan Plus 0.75mg/ml – 173mg/kg |
-21.35 |
-30.1138779 |
-12.5861221 |
0.0000043 |
|
Diamizan Plus 0.75mg – 217mg/kg |
-14.70 |
-23.4638779 |
-5.9361221 |
0.0005646 |
Table 5: Tukey Multiple Comparisons of Means of Trypanosomal Load at 95% Family-Wise Confidence Level –for Day One
Trypanosomal Load at Day Two:
At 48 hours of treatment, the trypanosomal load was highest in group 2 (173 mg/kg of the toad venom) followed by group 3 (217 mg/kg of the toad venom), then group 1 (130 mg/kg of the toad venom), whereas it was least in group 6 (0.75 mg/ml of Diamizan plus). Thus, there was a very significant difference (F20 = 107, Adjusted R2 = 0.9464, P < 0.00001, Figure 6) in the mean of trypanosomal load in Swiss mice at 48 hours of treatment with varying concentrations of toad venom and Diamizan plus drug. Trypanosomal load in Swiss mice in relation to multiple comparison of means between treatments at day 2 showed a significant difference (P < 0.0001) as shown in the Table 6.
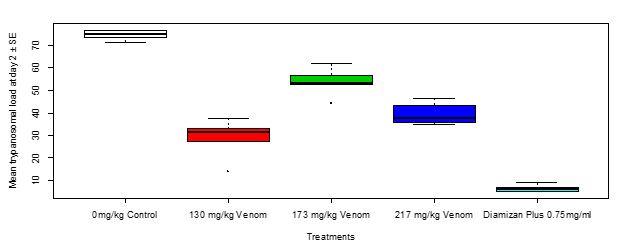
Figure 6: Mean trypanosomal load in Swiss mice in relation to treatments with toad venom and Diamizan plus treatments at day 2
|
Treatment |
Diff |
Lower |
Upper |
P – Adjusted |
|
130mg/kg – 0mg/kg |
-46.02 |
-56.5505483 |
-35.489452 |
0.0000000 |
|
173mg/kg – 0mg/kg |
-20.97 |
-31.5005483 |
-10.439452 |
0.0000705 |
|
217mg/kg – 0mg/kg |
-35.07 |
-45.6005483 |
-24.539452 |
0.0000000 |
|
Diamizan Plus 0.75mg/ml – 0mg/kg |
-68.32 |
-78.8505483 |
-57.789452 |
0.0000000 |
|
173mg/kg – 130mg/kg |
25.05 |
14.5194517 |
35.580548 |
0.0000061 |
|
217mg/kg – 130mg/kg |
10.95 |
0.4194517 |
21.480548 |
0.290893 |
|
Diamizane Plus 0.75mg/ml – 130mg/kg |
-22.30 |
-32.8305483 |
-11.769452 |
0.0000313 |
|
217mg/kg – 173mg/kg |
-14.10 |
-24.6305483 |
-3.569452 |
0.0055509 |
|
Diamizan Plus 0.75mg/ml – 173mg/kg |
-47.35 |
-57.8805483 |
-36.819452 |
0.0000000 |
|
Diamizan Plus 0.75mg – 217mg/kg |
-33.25 |
-43.7805483 |
-22.719452 |
0.0000001 |
Table 6: Tukey Multiple Comparisons of Means of Trypanosomal Load at 95% Family-Wise Confidence Level for Day Two
Trypanosomal Load at Day Three:
Group 2 (173 mg/kg toad venom) at 72 hours of treatment had the highest trypanosomal load followed by group 1 (130 mg/kg of the toad venom), then group 3 (217 mg/kg of the toad venom) whereas it was least in group 4 (0.75mg/ml of Diamizan plus).Hence, mean trypanosomal load in Swiss mice in relation to treatments with toad venom and Diamizan plus drug at day three of treatment had a very significant difference (F20 = 145.2, Adjusted R2 = 0.9601, P < 0.00001, Figure 7). Trypanosomal load in Swiss mice in relation to multiple comparison of means between treatments at day 3 showed a high significant difference (P < 0.0001) with the exception of between 130 mg/kg and 217 mg/kg as shown in the Table 7.

Figure 7: Mean trypanosomalload in Swiss mice in relation to treatments with toad venom and Diamizan plus treatments at day 3
|
Treatment |
Diff |
Lower |
Upper |
P – Adjusted |
|
130mg/kg – 0mg/kg |
-48.45 |
-59.01312 |
-37.886875 |
0.0000000 |
|
173mg/kg – 0mg/kg |
-23.00 |
-33.56312 |
-12.436875 |
0.0000214 |
|
217mg/kg – 0mg/kg |
-49.50 |
-60.06312 |
-38,936875 |
0.0000000 |
|
Diamizan Plus 0.75mg/ml – 0mg/kg |
-79.60 |
-90.16312 |
-69.036875 |
0.0000000 |
|
173mg/kg – 130mg/kg |
25.45 |
14.88688 |
36.013125 |
0.0000051 |
|
217mg/kg – 130mg/kg |
-1.05 |
-11.61312 |
9.513125 |
0.9981429 |
|
Diamizan Plus 0.75mg/ml – 130mg/kg |
-31.15 |
-41.71312 |
-20.586875 |
0.0000002 |
|
217mg/kg – 173mg/kg |
26.50 |
-37.06312 |
-15.936875 |
0.0000028 |
|
Diamizan Plus 0.75mg/ml – 173mg/kg |
-56.60 |
-67.16312 |
-46.036875 |
0.0000000 |
|
Diamizan Plus 0.75mg – 217mg/kg |
-30.10 |
-40.66312 |
-19.536875 |
0.0000004 |
Table 7: Tukey Multiple Comparisons of Means of Trypanosomal Load at Day 3
Change in Body Weight of Swiss mice after Treatment with Toad Venom and Diamizan Plus:
After treatment with the toad venom crude extract of different dosages and Diamizan plus drug, change in body weight was highest in Group 4 treated with 0.75 mg/ml of Diamizan plus, followed by Group 3 treated with 217 mg/kg of toad venom, then Group 1 treated with 130 mg/kg of toad venom whereas it was least in Group 2 treated with 173 mg/kg of the toad venom. Thus, the mean change in body weight of Swiss mice after treatment in relation to toad venom as well asDiamizan plus drug respectively showed a high significant difference (Kruskal-Wallis c2= 15.779, df = 4, P = 0.00333, Figure 8).The multiple comparisons of means of change in body weight between 173 mg/kg treatment and Diamizan plus drug showed a significant difference (P< 0.05, Table 8).
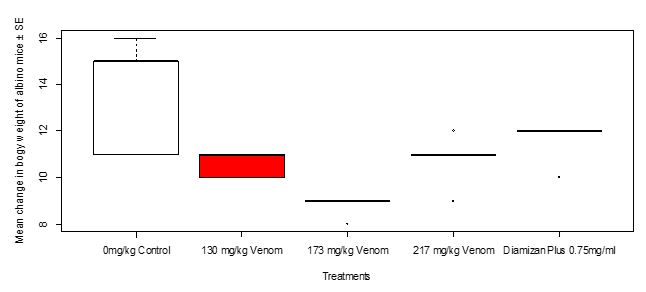
Figure 8: Mean change in body weight of Swiss mice after treatment with toad venom and Diamizan plus drug
|
Treatments |
||||
|
|
0mg/Kg |
130mg/Kg |
173mg/Kg |
217mg/Kg |
|
130mg/Kg |
0.087 |
0 |
0 |
0 |
|
173mg/Kg |
0.031 |
0.031 |
0 |
0 |
|
217mg/Kg |
0.168 |
0.555 |
0.060 |
- |
|
Diamizan Plus 0.75mg/ml |
0.428 |
0.128 |
0.031 |
0.219 |
P Value Adjustment Method: BH
Table 8: Tukey Multiple Comparisons of Means of Change in Body Weight Between Treatments
Change in Haematological Parameters of Swiss mice after Three Days of Parasitemia Treatment:
Hemoglobin (Hb):
After treatment with the toad venom crude extract of different dosages and Diamizan plus drug, the result showed that there was no change in the blood concentration of hemoglobin of the Swiss mice in the different groups. However, the mean change in Hb level in Swiss mice after three days of trypanosomal treatment between dosages of toad venom and Diamizan plus drug showed no significant difference (Kruskal-Wallis c2 = 8.141, df = 4, P = 0.08655, Figure 9).
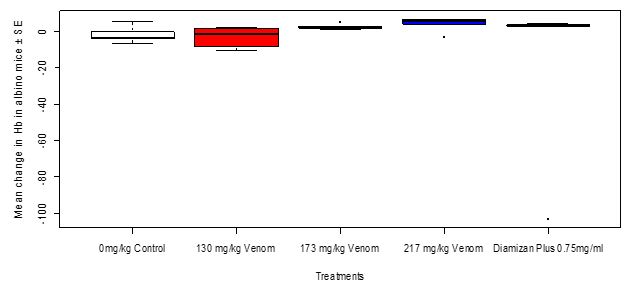
Figure 9: Mean change in hemoglobin level in Swiss mice in relation to treatments with toad venom and Diamizan plus drug after three days of trypanosomal inoculation
RBC
After treatment with the toad venom crude extract of different dosages and Diamizan plus drug, the result showed a change in the RBC level of the Swiss mice in the different treatment groups where group 4 treated with 0.75 mg/ml had the highest level followed by group 3 treated with 217 mg/kg of the toad venom crude extract then, group 2 treated with 173 mg/ml of the toad venom crude extract and group 1 treated with 130 mg/kg was the least. Thus, the mean change in RBC level in Swiss mice after three days of trypanosomal treatment between dosages of toad venom and Diamizan plus drug showed a high significant difference (kruskal-wallis c2 = 14.326, df = 4, P = 0.006324, Figure 10). Table 9 shows that multiple comparisons of means of change in RBC level between treatments between dosages of toad venom and Diamizan plus drug showed a high significant difference (P= 0.006324).

Figure 10: Mean change in RBC level in Swiss mice in relation to treatments with toad venom and Diamizan plus drug after three days of trypanosomal inoculation
|
|
Treatments |
|||
|
|
0mg/Kg |
130mg/Kg |
173mg/Kg |
217mg/Kg |
|
130mg/Kg |
0.526 |
0 |
0 |
0 |
|
173mg/Kg |
1.000 |
0.079 |
0 |
0 |
|
217mg/Kg |
0.099 |
0.060 |
0.099 |
0 |
|
Diamizan Plus 0.75mg/ml |
0.136 |
0.060 |
0.079 |
0.750 |
P Value Adjustment Method: BH
Table 9: Turkey Multiple Comparisons of Means of Change in RBC Level between Treatments
PCV:
After treatment with the toad venom crude extract of different dosages and Diamizan plus drug, the result showed a change in the PCV level of Swiss mice in the various treatment groups where group 3 treated with 217 mg/kg of the toad venom crude extract had the highest PCV level, followed by group 4 treated with 0.75 mg/ml of the Diamizan plus drug, then group 2 treated with 173 mg/kg of the toad venom and Group 1 treated with 130 mg/kg of the toad venom had the lowest. Thus, the mean change in PCV level in Swiss mice after three days of trypanosomal treatment between dosages of toad venom and Diamizan plus drug showed a very high significant difference (Kruskal-Wallis c2 = 18.513, df = 4, P = 0.0009793, Figure 11). Table 10shows multiple comparisons of means of change in PCVlevel between treatments which the highest dosage of toad venom versus Diamizan plus drug showed no significant difference (P = 0.317).
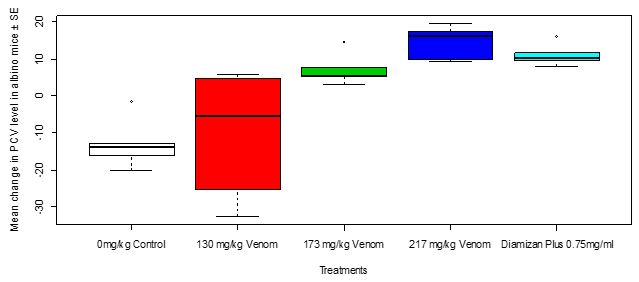
Figure 11: Mean change in PCV level in Swiss mice in relation to treatments with toad venom and Diamizan plus drug after three daysof trypanosomal inoculation
|
|
Trypanosoma Load and Treatments |
|||
|
|
0mg/Kg |
130mg/Kg |
173mg/Kg |
217mg/Kg |
|
130mg/Kg |
0.841 |
0 |
0 |
0 |
|
173mg/Kg |
0.026 |
0.136 |
0 |
0 |
|
217mg/Kg |
0.026 |
0.026 |
0.053 |
0 |
|
Diamizan Plus 0.75mg/ml |
0.032 |
0.032 |
0.139 |
0.317 |
P Value Adjustment Method: BH
Table 10: Turkey Multiple Comparisons of Means of Change in PCV Level Between Treatments
Discussion:
Bioactive Compounds Present in Bufonidae Toad Venom Crude Extract:
Paratoid secretions of toads have a high chemical complexity. They have diverse types of biomolecules as recorded in this study, such as proteins, chemical components and elements, peptides, biogenic amines, and alkaloids and other unidentified classes. Bufadienolides has several activities against bacteria, fungi, protozoa, virus, HIV, and cancer cells that have been described for this type of secretion (Preusser et al., 1975; Zhao et al., 2005; Bhattacharjee et al., 2011; Calderon et al., 2011).
Biochemical Compounds Present in the Toad Venom Crude Extract:
Using GC-MC techniques, compounds identified in this study like n-Hexadecanoic acid; 9,12-Octadecadieonic acid (Z,Z); 9,17-Octadecadienal (Z); Hexadecanoic acid 2-hydroxy-1-(hydroxymethyl) ethyl ester, are in harmony with the findings of Ajanaku et al. (2019) who reported that the methanolic extract of the leaf of C. adansonii having the same compounds, has cancer preventive, anti-microbial, anti-fungal and other therapeutic properties which could suppose that toad venom crude extract equally possesses anti-trypanosomal therapeutic potentials as observed by Aparna et al.(2012) and Sanni and Omotoyinbo (2016).
The Protein and Non-Protein Functional Groups Present in The Toad Venom Crude Extract:
According to Sakate and Oliveira (2000), the substances that make up the toad venom can be divided into basic compounds (biogenic amines) and steroid derivatives. Consequently, the biochemical analysis using FTIR procedures employed in this study revealed the presence of amines like aniline and triethylamine which is in agreement with the earlier discoveries of Andrade et al. (1998) who reported that amino acids and polyamines are established membrane transporters in T. cruzi. These findings apparently suggest that the amino-group (aniline and triethylamine) were easily absorbed by T. brucei brucei and could be potential therapeutic targets due to the anti-trypanosomal activity of the toad venom crude extract (Melisa et al. 2019).Tempone et al. (2008) reported antiparasitic activity of the steroids isolated from toad venom crude extract and only hellebrigenin from the biogenic amines was active against trypomastigote of T. cruzi, which could be attributed to the activity against T. brucei brucei as seen in this study.
The Chemical Elements Present in the Toad Venom Crude Extract:
Except for lead (Pb) and aluminium (Al), every other element identified in this study using SEM-EDS are essential for the human body, and this agrees with the findings of Lingamaneni et al., (2015) who stated that these elements functionin the stabilization of cellular structures (immune systems) at normal levels but in deficiency, may stimulate alternate pathways and cause diseases thus, these elements could be considered to be chemotherapeutic and chemo-preventive agents which stimulate the immune systems in the treatment of other diseases and may be, trypanosomal infection as demonstrated in this study.However, variation in the toad’s diet can influence the molecules uptake by feeding, modifying the secretion composition and activity as well, and this requires further and sustained research in different places, looking for constituents of toad venoms from various species (Hantak, et al., 2013).
Oral Toxicity (LD50) of the Toad Venom Crude Extract on the Laboratory Swiss Mice:
Prior to the mortality of the laboratory animals, symptoms such as hyperactivity, convulsion and constant stooling were observed which suggests that the toad venom extract was lethal and even up to 1000 mg/kg after an hour, however on the administration of 750 mg/kg, hyperactivity, diarrhea and sedation were observed within one hour, but there was no mortality recorded. Following the Lorke’s method computation, the oral toxicity of the toad crude venom extract was established as 866 mg/kg. This finding agrees with Tubaro et. al. (2011) who investigated the acute oral toxicity of a new palytoxin congener in mice which induced scratching, jumping, respiratory distress, cyanosis, paralysis and death in mice within 24 hours but not in accordance with Al-Afifi et. al. (2018) who reported that Dracaena cinnabari resin methanol extracts in rats, showed no treatment related mortality.
Pre-patency period of Trypanosoma brucei brucei:
The lack of variation observed at day zero prior to treatment in the mean of trypanosomal load in Swiss mice in relation to treatments with toad venom and diamizan plus drug respectively could be because the Swiss mice were yet to receive any form of treatment at this stage. The results possibly suggest that the pre-patent period of Trypanosoma brucei brucei was 3 days, this is in line with the finding by Turay et al. (2005) and Udensi and Fagbenro-Beyioku (2012).
Trypanosomal Load in Swiss Mice in Relation to Treatments with Toad Venom and Diamizan Plus Drug:
The observed variation in the three (3) days pooled trypanosomal load in Swiss mice in relation to treatments possibly suggests that the standard drug (diamizan plus) is effective. This is in consonance with the finding of Ezeh et al.(2016) who reported that treatment with Diminazene aceturate (Berenil) resulted in the reduction of parasitemia load in infected mice after two days of treatment. Although the toad venom did not actively reduce the Trypanosomaparasitaemia as much as diamizan plus drug did when both of their mean load was compared, however, the highest dosage of toad venom treatment still yielded over 50% reduction in trypanosomal load in Swiss mice in comparison with the control group. This suggests that the toad venom has the potential to relatively reduce trypanosomal load in the Swiss mice which corroborates with Tempone et al. (2008) who reported antiparasitic activity of steroids (telocinobufagin and hellebrigenin) from toad venom crude extract and also leishmanicidal activity against L. infantum promastigotes with IC50 of 126.2 and 61.2