Aditum Journal of Clinical and Biomedical Research
OPEN ACCESS | Volume 8 - Issue 1 - 2026
ISSN No: 2993-9968 | Journal DOI: 10.61148/2993-9968/AJCBR
Natalia Aguirre 1 , Gastón Martínez 1 ,Aldo Sgaravatti 2 ,Marta Vázquez 1 and Cecilia Maldonado 1* 1Pharmaceutical Sciences Department. Faculty of Chemistry, Universidad de la República, Montevideo, Uruguay.
2 dirección Nacional de Sanidad Policial, Hospital Insp Gral Ubaldo Genta, Montevideo, Uruguay.
*Corresponding Author: Cecilia Maldonado, Pharmaceutical Sciences Department. Faculty of Chemistry, Universidad de la República, Montevideo, Uruguay.
Received: July 17, 2021
Accepted: July 22, 2021
Published: July 28, 2021
Citation: N.Aguirre, G.Martínez, A.Sgaravatti, Vázquez.M and Maldonado.C. (2021) “Managing Older Adults with Polypharmacy: Considerations from The Perspective Pharmacy Students.”, Aditum Journal of Clinical and Biomedical Research, 3(1); DOI: http;//doi.org/07.2021/1.1053.
Copyright: © 2021 Cecilia Maldonado. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
The incorporation of Pharmaceutical Care in Pharmacy students’ curricula has given future pharmacists the opportunity to interact with health care users and professionals. Prescribing cascades are one of the main problems for elderly patients, leading to polypharmacy and adverse drug reactions (ADR).
Objective: To present the study of an elderly patient pharmacotherapy in the context of pharmacy experiential education that involved discussion with the geriatrician.
Materials and Methods: A 72-year-old woman who was receiving 19 drugs was interviewed by pharmaceutical care students and her pharmacotherapy studied using STOPP/START criteria.
Results: Interaction among students, patient and geriatrician redounded in a thorough analysis of the pharmacotherapy. Most of the drugs were prescribed to treat possible ADRs and that the patient presented several interactions together with symptoms of an anemic state.
Conclusion: Students had the opportunity to interact with patients, putting into practice tools that have proved to be useful to detect possible inappropriate prescription. Discussion with the geriatrician improved the outcome by enriching the results of an interdisciplinary approach. In this case, because of many prescribing cascades, the patients’ pharmacotherapy resulted in an intricate connection of prescriptions
Introduction:
Pharmacy students in Uruguay have little chances to interact with patients and health care professionals on a regular basis, this lays on different reasons, being one of them the curricula. The inclusion of Pharmaceutical Care as a subject in the studying plan has given the students the opportunity to relate to patients, physicians and nurses. In the health setting professors of two Departments, Pharmaceutical Sciences and Geriatrics, have started collaborating to introduce students in the use of different tools to evaluate elderly patients in order to optimize their therapy.
Elderly patients are always a challenge for the health care system. In 1965 Bernard Isaacs described 5 giants in geriatrics: immobility, instability, incontinence, and impaired intellect/memory [1]. Over the years these concepts have evolved to the four syndromes: frailty, sarcopenia, the anorexia of aging and cognitive impairment [2,3,4,5]. These conditions lead patients to falls, hip fractures, depression, and delirium [6,7,8,9]. The early detection of these syndromes can reduce disability, hospitalization, institutionalization, and mortality 10. What needs to be understood is that polypharmacy is one of the factors that may precipitate the beginning of any of these syndromes.
Prescribing cascades usually occur when signs and symptoms of a patient are inaccurately assessed and an adverse drug reaction (ADR) is misinterpreted as a new condition, resulting in a new medication being prescribed, leading to polypharmacy [11,12]. In the last decades, polypharmacy, defined here as the consumption of 5 or more drugs for at least 6 months 13, has become an increasingly frequent problem among elderly population. This is due to demographic and socio-sanitary factors such as gender, cultural level, economic position and access to primary health care, fragmentation of the health care system, multiple prescribers, and ineffective doctor-patient communication among others. Furthermore, on a biological level, aging produces a series of physiological changes that are associated with alterations in drug pharmacokinetics and pharmacodynamics making older adults more vulnerable to ADRs [14]. Polypharmacy has been associated with an increased risk of potentially inappropriate prescribing of medication and of ADRs that may cause falls and geriatric syndromes [15, 16]. A useful strategy to evaluate the appropriateness of a prescription and to avoid polypharmacy in the elderly population is to use the STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions) and START (Screening Tool to Alert to Right Treatment) criteria [17,18].
Pharmacotherapy’s study to detect possible prescribing cascades is a demanding activity and the collaboration of different professions profiles enriches the process of discussion. We will describe a case that illustrates the clinical perils of polypharmacy and serves as a point for critical discussion of pharmacy students.
Methods:
Form After completion of the theoretical part of the subject Pharmaceutical Care, pharmacy students performed a pharmacotherapeutic interview, following a form previously designed for this purpose during class. The form included information about patient’s illnesses, pharmacotherapy, and medicinal plants consumption. Laboratory results were collected from medical chart. To assess patient’s therapy STOPP-START criteria was used to find potentially inappropriate prescriptions and omissions. Other aims of the study included: identifying prescribing cascades, ADR detections and drug interactions. Their analysis was then discussed with the geriatrician and pharmacy professors. What follows are their findings regarding the criteria applied and a discussion of the pharmacotherapy evaluated.
Results:
M.C.C was a 72-year-old Caucasian woman who attended a medical appointment with the geriatrician for the first time. Her multiple medical conditions included type II diabetes, hypothyroidism, depression, chronic stress, gastritis, H Pylori, duodenitis, hiatal hernia, irritable bowel, osteoarthritis, urinary incontinence. She had been treated for depression for over 40 years and attempted to kill herself on one occasion. Anemia was diagnosed in consultation; hemoglobin level was 11.8 g/dL and iron and ferritin levels were determined after consultation. She also referred fear of falling because of having suffered from frequent falls which led to multiple fractures and loss of dental pieces.
M.C.C was currently taking 19 drugs which included: metformin, oxybutynin, gliclazide, T4, lamotrigine, escitalopram, betahistine, vitamin B12, domperidone, loratadine, zolpidem, atorvastatin, tizanidine, pinaverium, gabapentin and long-standing prescription of alprazolam, ibuprofen, diclofenac and omeprazole (for the past 30 years).
Table 1 displays the STOPP and START criteria present in this clinical history and the medication related to them.
|
STOPP CRITERIA |
||
|
A1 |
Any medication prescribed without an indication based on clinical evidence |
Atorvastatin, gabapentin, tizanidine, ibuprofen, diclofenac |
|
A2 |
Any medication prescribed for a longer duration than indicated |
Omeprazole, alprazolam, ibuprofen, diclofenac |
|
A3 |
Duplicate drug classes |
Ibuprofen, diclofenac |
|
D5 |
Long-term prescription of benzodiazepines (>4 weeks) |
Alprazolam |
|
F3 |
Drugs that usually cause constipation in patients with chronic constipation |
Oxybutynine |
|
J1 |
Long-acting sulfonylureas with diabetes mellitus type II |
Gliclazide |
|
K1 |
Benzodiazepines in those prone to falls |
Alprazolam |
|
K4 |
Z-drugs in those prone to falls |
Zolpidem |
|
N1 |
Concomitant use of 2 or more drugs with antimuscarinic / anticholinergic properties |
Oxybutynin, tizanidine |
|
START CRITERIA |
||
|
E3 |
Calcium and vitamin D supplement in patients with known osteoporosis |
|
|
E5 |
Vitamin D supplements in elderly patients prone to falls |
|
|
Table 1: STOPP/START criteria relevant to the case. |
||
It is noteworthy that neither the patient nor the medical chart referred to high cholesterol or neuropathic pain so the inclusion of atorvastatin and gabapentin in the patient’s medication has no clinical evidence. Diclofenac, ibuprofen and tizanidine may be present as Over-the-counter medicines (OTCs) for the treatment of some painful condition but no prescribed by the clinicians.
Duration of treatment raised concern regarding benzodiazepines, not only because of the patient’s age but also taking into consideration that the patient is prone to falls, and the effect of alprazolam is added to the one of zolpidem.
This case also illustrates the dynamics of a drug prescription cascade, which eventually resulted in intricated interconnections of prescriptions. Figure 1 illustrates how this can be perceived as a prescribing web.
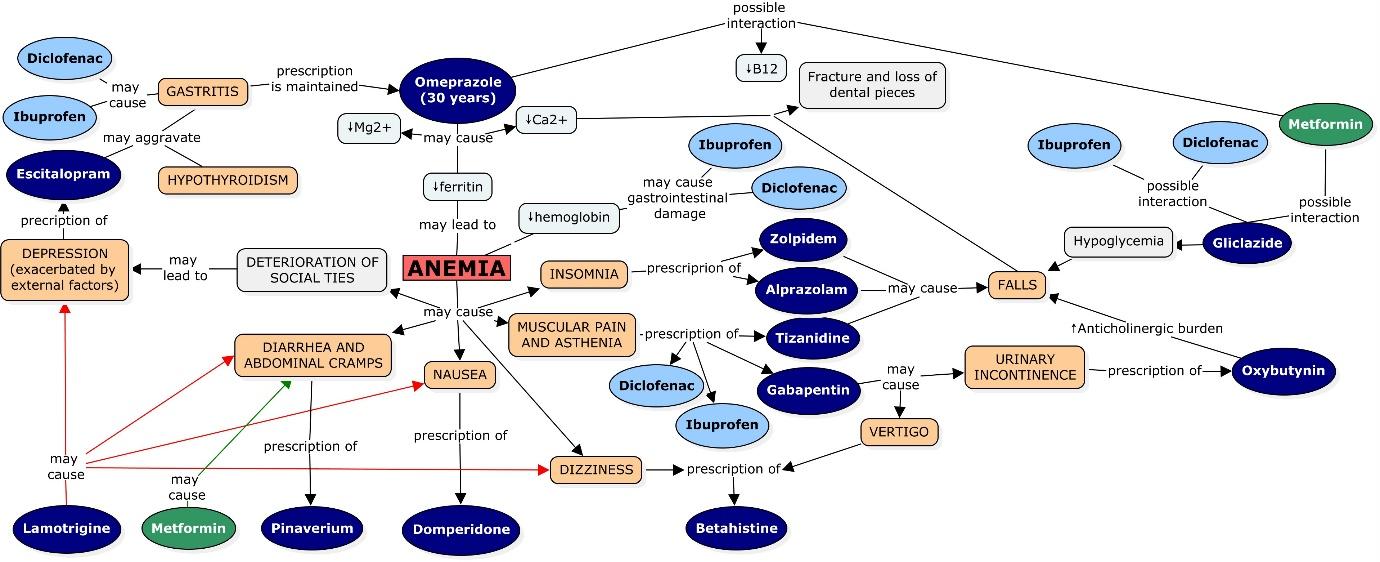
Figure 1: This figure illustrates the intricated pathways that may have led to a prescribing cascade that transformed in time into a prescribing web.
In order to analyze the cycle shown in Figure 1, we decided to use omeprazole as a starting point as the patient had been taking the drug for more than thirty years. Studies have shown that taking omeprazole for long periods of time can result in depletion of ions such as magnesium, calcium and iron by impairing its absorption because of changes in stomach pH [19,20].
Regarding iron it is known that it can either be absorbed forming a chelate with heme group or in its ionic form found in salts. Ionic iron absorption is affected by several factors such as the gastric pH, an acidic pH facilitates iron dissolution by forming soluble chelates, and by the reduction of Fe (III) to Fe (II). It has been reported that achlorhydria patients present a lower intestinal absorption of iron in its salts [21]. Therefore, omeprazole may have a negative influence on the absorption of this ion. Patients with iron-deficiency should be supplemented with higher doses of iron salts while taking proton pump inhibitors [22].
The administration of ibuprofen and diclofenac should also be taken into consideration as non-steroidal anti-inflammatory drugs (NSAIDs), especially when used for long periods of time, may cause bleeding through gastrointestinal damage, lowering hemoglobin provoking iron deficiency as well [23].
There have also been reports of anemia related to omeprazole induced by deficiency of vitamin B12 [24,25], in this case the patient had been supplemented with this vitamin but still vitamin B12 level, 156.8 pg/mL, was below population range, 197-771 pg/mL, The depletion of this vitamin can be aggravated by the concomitant use of metformin which also diminishes vitamin B12 levels by interfering with its absorption [26,27].
However as stated before the mechanisms by which omeprazole exert this adverse effect are multiple and vitamin supplementation may not revert all.
The patient’s low level of ferritin, 24 ng/mL, (population range 30-400 ng/mL) evidenced low iron stores in the body, and this low iron stores maintained in time may result in anemia [28,29]. In the case of this patient the combination of omeprazole, ibuprofen and diclofenac may have contributed to the anemia which was not reverted by the solely use of vitamin B12 as other mechanism underlie the ion depletion.
Like iron, magnesium salts depend on gastric pH to dissolve; hence increase of gastric pH caused by omeprazole may reduce its absorption [30].
Omeprazole has also been reported to increase the risk of fracture [31,32,33]. One possible mechanism that has been proposed was the interference with calcium absorption [33,34]. This assumption is based on the fact that the majority of dietary calcium is in the form of relatively insoluble salts, which means that gastric pH needs to be acid to facilitate a higher dissolution, and therefore a better absorption 21. Nevertheless, controversial results were found when studies with patients were performed. Some of them showed that omeprazole had no effect on calcium absorption while others yielded the opposite result [33,34]. For example, Graziani et al reported 35 that achlorhydria reduces the absorption of calcium, increasing the risk of osteoporosis. Additionally, it is important to consider that the stomach secretion of chlorhydric acid is reduced throughout the years, and it decreases considerably after the age of 60 [35]. Magnesium and calcium levels were not determined at the moment of the interview so data about these two ions is missing.
Insomnia, asthenia and muscular pain, dizziness, nausea, diarrhea, abdominal cramps and deterioration of social ties were some of the symptoms associated with anemia that were reported by the patient to different doctors. However, these symptoms were not correlated with the disease until her first appointment with the geriatrician. Due to this fact, most of the drugs that the patient had been taking aimed to treat or alleviate some of the symptoms mentioned above [36,37,38,39].
In order to treat insomnia zolpidem and alprazolam were prescribed, drugs that according to the STOPP/START criteria may increase the risk of falls in elderly patients, taking into consideration that the patient referred fear of falling due to previous episodes the maintenance of these medication should be a compromise between risks and benefits.
Furthermore, M.C.C was receiving metformin and gliclazide to treat her diabetes mellitus, as well as ibuprofen and diclofenac to treat asthenia. The concomitant use of these drugs may present pharmacokinetic and pharmacodynamic interactions leading to hypoglycemia and increasing the risk of falls [40, 41].
As a way to treat the asthenia and the muscular pain apart from the two different NSAIDs, gabapentin and tizanidine were also included in the patient’s therapy. Tizanidine may increase the risk of falls, meanwhile gabapentin presents as a potential side effect urinary incontinence, a problem reported by the patient [42,43]. Oxybutynin, an anticholinergic medication, was prescribed in order to relieve her inability to control urination provoking an even more significant increase in the risk of fall.
In order to reverse dizziness, drowsiness and vertigo provoked by gabapentin, and the anemia itself, betahistine was included in the therapy.
As mentioned, the patient presented nausea and gastrointestinal disorders, for which she received domperidone and pinaverium respectively. Nevertheless, once anemia was diagnosed, those symptoms were attributed to the disease rather to a new pathology.
In the patient’s case, the deterioration of the social ties as a consequence of anemia was manifested as general apathy, reluctancy to be engaged in social activities which over time led to depression, a problem that was accentuated by external factors. Hence, escitalopram was included in her therapy. This drug may increase the risk of bleeding, particularly in the gastrointestinal tract, such as other serotonin reuptake inhibitors (SSRIs). This adverse effect is explained by the fact that platelets use serotonin as an aggregation factor. Therefore, by inhibiting its reuptake, escitalopram reduces their aggregation capacity, increasing the risk of bleeding [44]. This risk acquires more significance with concomitant use of NSAIDs 44. In this case the patient developed a gastrointestinal bleeding, worsening her anemia status.
Apart from that, NSAID-Induced gastropathy results in the need of maintaining omeprazole in the therapy [45], transforming the prescribing cascade into a prescribing web.
Pharmacotherapy analysis was then discussed with the geriatrician who commented and added its impression to the evaluation performed by the students, from the results presented results evident that students were able to perform a thorough analysis of the patient’s pharmacotherapy, familiarizing with some validated tools that enable primary analysis of polypharmacy that should then be complemented with more data from the clinical history of the patient.
Conclusions:
Managing polypharmacy in the elderly is a complex process. The application of STOPP/START criteria allowed students to identify potentially inappropriate prescriptions, which in addition to the drug's pharmacological knowledge, led to hypothesize that some of the patient’s health problems which were pharmacologically treated, were actually due to adverse reactions to other drugs.
This is a challenging case that was studied from a multidisciplinary perspective, this approach redounds in an improvement geriatric patient care, preventing unnecessary medications as well as differentiating adverse drug reactions from the symptoms of a developing illness.
Funding: This work received no funding.
Author’s Contribution:
Data collection and first analysis was performed by Natalia Aguirre and Gastón Martinez. Natalia Aguirre designed the figure. Marta Vázquez, Aldo Sgarabatti and Cecilia Maldonado completed the final analysis and revision.
Conflicts of Interest: The authors have no conflicts of interest to declare