Aditum Journal of Clinical and Biomedical Research
OPEN ACCESS | Volume 8 - Issue 1 - 2026
ISSN No: 2993-9968 | Journal DOI: 10.61148/2993-9968/AJCBR
Rajiv Kumar
NIET, National Institute of Medical Science, India.
*Corresponding Author: Rajiv Kumar, NIET, National Institute of Medical Science, India.
Received: June 17, 2021
Accepted: June 21, 2021
Published: June 28, 2021
Citation: Rajiv Kumar. (2021) “Healing, and Repair of Inflammatory Induced Injuries: Routes of Rejuvenation.”, Aditum Journal of Clinical and Biomedical Research, 2(5); DOI: http;//doi.org/06.2021/1.1048.
Copyright: © 2021 Rajiv Kumar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
In the mechanisms of repair, and regeneration, countless cellular events, including chemical, biochemical, electrochemical, resident, and circulating cells take part and deal with mechanical signals initiated at the ECM tightly. These interlinked transformations coordinated for efficient repair of damaged tissue. Their involvements are there in a cohesive action of many cell types, the extracellular matrix, and soluble mediators that were notified as cytokines
Opinion:
In the mechanisms of repair, and regeneration, countless cellular events, including chemical, biochemical, electrochemical, resident, and circulating cells take part and deal with mechanical signals initiated at the ECM tightly. These interlinked transformations coordinated for efficient repair of damaged tissue. Their involvements are there in a cohesive action of many cell types, the extracellular matrix, and soluble mediators that were notified as cytokines.[1] Immediately after injury, a plug of cellular debris forms, and then the injury underway darkens, which restricts the bleeding by forming a protective layer, culminating in the restoration of tissue integrity. These cellular events are the initial activities of mechanisms of repair and regeneration.[2] The coagulation and hemostasis processes are crucial events, which stop bleeding, acts as an effective barrier. It forms a tough scab and prevents blood loss, further, it also protects tissues from any infection.[3] Labeling of the nuclei is a major event of healing wherein epidermal cells apply a fluorescent protein to them staining their membranes.[4] After an injury, cells elongated toward the affected part, aligned themselves, then formed a large multinucleate epidermal cell and edging the scab. These cells re-establishing a continuous epidermis by spreading along and through the plug, and governed by the c-Jun N-terminal kinase (JNK) signaling pathway that affects the genetics and cellular events of restorative features.[5] Various cellular and molecular events such as mechanical homeostasis, cellular, and molecular aspects of dysfunctional mechanotransduction, relevant therapeutic strategies influenced the processes of healing of injuries. Despite it, several other phenomena and mechanisms take part in it, but it is not yet clear how dysfunctional mechanotransduction initiates diseases and irregular healing.[6] How does the cartilage extracellular matrix stimulus mechanical environment for restoring, mechanical homeostasis still unanswered?
The cell-matrix interactions initiate a mechanosignaling route for transmitting the mechanical signals from the matrix.[7] These mechanisms occurred on the cell surface and transformed intracellular responses via activation of chondrocyte mechanoreceptors.[8] For, example, a mechanical load governed multiple signaling cascades for maintaining cartilage homeostasis. The cytokines, soluble mediators are some critical components of injury healing and communicate activities to cells via the extracellular matrix.[9] The processes of degradation and regeneration involved many cell types and governed complex interactions between multiple biochemical cascades are present in chemotaxis.[10] Growth factors stimulate epithelial cells for proper growth and fibroblasts that initiate the formation of new blood vessels (angiogenesis).[11] Later on, several other mechanisms including stimulating matrix formation, proliferative (re-vascularisation, granulation, re-epithelialization, contraction), remodeling proceedings initiated by the several kinds of growth factors i.e. epidermal growth factor (EGF), transforming growth factor (TGF)-α, fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF).[12] The vascular cells endothelial, fibroblasts, and smooth muscle responds to mechanical stimuli to do changes in their gene expression profile and respond to do alternations in mechanics.[13] These aforementioned changes answered how vascular adaptations initiate perturbations in hemodynamics and convinced pathologies. As mechanical loading plays a key role in injury healing, inflammation also determines various routes of vascular adaptations and disease progression. The interactions between mechanobiological and immunobimmunologics of vascular growth and remodeling also affect the profile of vascular health and disease.
Platelets have inbuilt self-healing mechanisms and capable of clinically manipulate cellular routes and events, and thus can trigger tissue repair.[14] For example, the use of prolotherapy to treat musculoskeletal pain, related injuries, and rejuvenation of dermal tissue. Platelet-derived growth factors promote tissue repair events and routes, including chemotaxis, remodeling, wound extracellular matrix deposition, cell proliferation, and angiogenesis.[15] For a promising therapeutic modality, Platelet-derivatives as regenerative medicine offers enormous openings for treating injuries and soft-tissue injuries.[16] Platelet activation binds adhesive ligands of the excitatory platelet agonists and in the meantime its cognate receptors on its membrane for enhancing the frequencies of intracellular signaling (Fig. 1). These phenomena stimulate some features of tethered platelets. At the same, the phenomena of de-granulation in the platelets, releases warehoused secondary agents (ADP, ATP, and synthesize thromboxane) and as a result, platelet aggregation occurred. Later on, the process of activation of alpha(IIb)beta(3) happened, and it promotes adhesion.[17] Overall, the rate of recurrence of platelet-platelet interactions increased, and then, the intracellular calcium percentage enriched.[18] These cellular activities stimulate transportation of negatively charged phospholipids for catalyzing to activate blood coagulation factors: factor VIII and factor I.[19] Growth factors and cytokines released by activating platelets stimulate cells to start re-epithelialization, connective tissue repair, and angiogenesis.
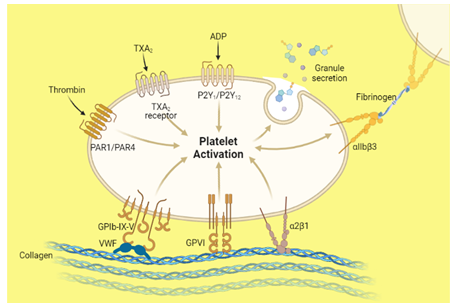
Figure 1: Illustrate the phenomenon and role of platelet activation. “Adapted and created with permission from [biorender.com] and acknowledged
Initiated factors, neutrophil, macrophage influx, and bacterial components generate inflammatory responses for promoting these inflammations to eradicate the bacteria. Therefore, inflammation effectively enhanced healing routes. Quickly ensued re-epithelialization ensures a better barrier functioning via neutrophil-macrophage interactions. The influx of macrophages having specific cells with an ability to release pro-inflammatory cytokines.[20] Simultaneously, reparative macrophages stimulate granulation tissue formation, remodeling, and angiogenesis.[21] The phenomenon and cellular events initiate fast healing for fast recovery. Macrophages promote the differentiation of resident cells to generate fibroblasts. This newly originated biological cell synthesizes the extracellular matrix and collagen.1 This structural framework plays a critical role in wound healing by producing a matrix via remodeling and turned to either scar in the skin or normal mucosal tissue.[22] Further, to get more information, a novel computational framework can analyze the route of the simulation and healing of an injury.
Acknowledgments:
Author (Rajiv Kumar) gratefully acknowledges his younger brother Bitto for motivation. The author acknowledges bio render for providing the facility to illustrate the diagrams (fig. 1) and again acknowledges the same.
Availability of data and materials:
Wherever necessary, relevant citations are included in the reference section. The author divided the reference section into two-part, scientific references and non-scientific references to avoid any confusion.
Competing interests:
The author has declared that no competing interest exists.