Aditum Journal of Clinical and Biomedical Research
OPEN ACCESS | Volume 8 - Issue 1 - 2026
ISSN No: 2993-9968 | Journal DOI: 10.61148/2993-9968/AJCBR
Kamal Prasad
Absolute Foods, Microbiology Division, 240-P, Sector-55, Gurugram-122011, Haryana, India
*Corresponding Author: Kamal Prasad, Absolute Foods, Microbiology Division, 240-P, Sector-55, Gurugram-122011, Haryana, India.
Received: May 29, 2021
Accepted: June 07, 2021
Published: June 16, 2021
Citation: Kamal Prasad. (2021) “Potential Impact of Seed Coating with Beneficial Microorganisms to Meticulousness Sustainable Organic Agriculture for Quality Nutritive Food Production for Modern Lifestyle, Improve Global Soil and Environmental Health towards Green Technology.”, Aditum Journal of Clinical and Biomedical Research, 2(4); DOI: http;//doi.org/06.2021/1.1043.
Copyright: © 2021 Kamal Prasad. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Seed coating (SC) could be a technique of casing seeds with adhesive agents to boost seed performance and germination whereas reducing cost. To meet the requirements of development of precision agriculture, SC has been widely utilized in agriculture as an effective means to alleviate biotic and abiotic stresses, therefore enhancing crop growth, productivity similarly as health. Plant beneficial microorganisms (PBM) such as plant growth promoting bacteria (PGPB), rhizobium, arbuscular mycorrhizal fungi (AM fungi), Trichoderma etc. can cut back the utilization of agrochemicals and increase crop yield, nutrition, and tolerance to biotic and abiotic stresses via direct use to the rhizosphere and plant tissues, or seed immunisation. However, during conventional inoculation processes (CIP), numerous factors such as insufficient microbial survival (MS), hindrance within the application of biocontrol inoculum to the seeds and exposure to unsuitable temperature and light in subsequent seed storage (SS), force us to explore economical and reliable microbial application tools. Typically, microbial seed coating (MSC) employing a binder/filler, mixed with inoculum, and can be done victimisation easy mixing equipment or a lot of specialized/sophisticated equipment. Binders/fillers can be accustomed extend microbial survival. The foremost reported types of SC are seed dressing, film coating, and pelleting. Microbial seed coating is promoting crop protection against pathogens and improving seedling establishment and germination or achieving high yields and food quality, below reduced chemical fertilization. The proper combination of biological management agents (BMA) applied as SC can be a powerful tool against a large number of diseases and pathogens. Recently, biological seed coatings (BSC) with plant growth promoting microorganisms (PGPM) are projected as alternative to traditional seed treatment because of its ecological safety and socio-economic aspects. During this manuscript, microbial seed coating technology and its contribution to sustainable precision organic agriculture are well mentioned.
Introduction:
The demand for biological seed coating (BSC) solutions is increasing in worldwide. They make sure that farmers defend their potential yield and quality by minimizing crop loss. Agriculture productivity in Indian sub-continent has gained encouraging treads throughout last four decades. High yielding seed varieties, avaibility of water for irrigation, maximum edges of biological and chemical fertilizers (CF) are the main factors for achieving high productivity. But the pathway adopted by us has been dependent on non-renewal energy resources, leading to associate exponential increase within the consumption of petroleum products (PP). Urea is the main CF being applied across the world in maximum quantities as compare to the other fertilizers. Excessive use of urea and different PP isn't solely valuable however conjointly unsafe for human health (HH) and environment. In view of sky rocking population and growing demand, the requirement of intensive agriculture is probably going to continue. In observation of the requirement of intensive agriculture and keeping economy, health, and environment in mind, the necessity of the hour is to export all attainable sources of plant nutrients, therefore, as to achieve the specified productivity through intensive agriculture. Microbial biofertilizers (MBF) are environment friendly, extremely effective, and low-cost agriculture inputs (LCAI) [1- 4]. The appropriate used of microbes on various crops are directly or indirectly a real service to the soil of nation and also the environments. Seed coating (SC) is that the better improve technology for correct use of microbes for productivity of crops [4-7].
Biological microbial inoculums (BMI) developed for SC on seeds for better self-life, germination and efficacy. It contains of biofertilizer and biopesticide cultures and plant growth promoting rhizosphere bacteria (PGPRB) [1, 6- 9]. Microbes are applied as SC to encourage germination, growth of seedlings, and for management of SC borne fungal diseases [6, 10]. Unhealthful fungal spores or bacteria can infect seeds whereas they're still developing on the plant and even when the harvest. These pathogens cause diseases within the next crop (seed-borne diseases). They successively cause diseases to consumers of those crop products. Therefore, antagonistic fungi or bacteria are used in SC to safeguard the seeds. Cereals, vegetables, oil seed crops, pulses are more and more being recognized for their role in promoting healthiness [11-14]. Researchers have reported that regular consumption of organic vegetables and pulses could cut back the chance of heart disease, diabetes and bound varieties of cancer. Vegetable and pulses are a flexible, easy to-prepare ingredient which can be used in entrees, salads, breads and desserts. The terms seed treatment (ST) and seed coating (SC) are typically considered same however these don't seem to be similar. ST can be defined as a way of treating seeds with some chemicals like fungicides, pesticides, insecticides, herbicides, and biological alone, with nonextra carriers however SC is value addition step of seed coating technology [15].
SC has been thought of as an explicit and cost-efficient technique (CET) to deliver microbial inoculants [14, 16-17], with the potential for large-scale application. SC could be a technique during which a lively ingredient is applied to the surface of the seed with the help of a binder and in some cases a filler which can act as a carrier. SC has been proposed as a promising tool for immunisation of various crop seeds, since it's able to use minor amounts of inoculum in an exceedingly precise application [1, 18-24]. The most types of SC include seed dressing (SD), film coating (FC), encrusting, pelleting etc., which might be chosen otherwise, according to the aim of application and therefore the form of seed or designated microbes. During this manuscript discussed SC as any technique during which the seed surface is roofed by materials (solid or liquid containing dissolved or suspended solids) forming a lot of or less continuous layer (physical barrier).
Plant Beneficial Microorganism’s (PBMS):
Microorganisms that benefit plant establishment, better germination, growth, and development by direct or indirect mechanisms are usually referred to as PBMS. Presently PGPB, Rhizobium, AM fungi, Trichoderma are most importance microbial inoculants in agro ecosystems. PBMS are thought about to be a natural alternative path to ease the pressure on the environment ensuing from conventional agriculture (CA). These microbes can facilitate plants maintain or increase productivity whereas reducing the input of agrochemicals, restoring soil fertility, and/or overcoming issues caused by abiotic and biotic stresses [3, 25-27].
Microbial Inoculation (MI):
PBMS are sometimes treated to the soil, the seed, seedling or the plant (foliar spray) [6-7, 28- 29]. Every immunization methodology has benefits and downsides, betting on the quantity of inoculants, availableness of apparatus, types of seed (size, shape, and fragility), the presence of inhibiting compounds within the seed (fungicides, micronutrients, and PBMS), and cost [30-31].
Plant Growth Promoting Bacteria (PGPB):
Bacteria are undoubtedly, the foremost plenteous microorganism’s present within the rhizosphere [32]. PGPB are bacteria that can enhance plant growth and protect plants from disease and abiotic stresses through a wide variety of mechanisms; those that establish close associations with plants, such as the endophytes, could be more successful in plant growth promotion. Numerous genera of bacteria such as genus Pseudomonas, Fraturia, Azospirillum, Azotobacter, Acetobacter, Rhizobium, Azospirillum Klebsiella, Enterobacter, Alcaligenes, Arthrobacter Bacillus, Serratia and Burkholderia contain species that have positive effects on plant growth and development. These helpful bacteria, additionally nominated as PGPB, are accountable for protective plants from biotic and abiotic stresses, enhancing plant growth and performance through direct and indirect mechanisms [7, 33-35]. PGPB can act as biofertilizers, phytostimulators, rhizoremediators, stress bio alleviators, bio modifiers, or biological control agents (BCAs) and biopesticides [1, 36-37].
Plant Growth Promoting Fungi (PGPF):
Momentous PGPF include species of the genera AM fungi, Aspergillus, Trichoderma, Penicillium, Piriformospora, Phoma, and Rhizoctonia, which have the ability to stimulate plant growth. AM fungi associated with the roots of virtually more than 95% of vascular plants and to live in symbiotic relationship [1-3, 27, 38]. These symbiotic associations are of great connexion for agricultural systems particularly below low input of agrochemicals, because of their role in increasing macro and micronutrients uptake and acquisition [1-4, 14, 19, 27, 38-39]. Moreover, AM fungi are able to improve soil aggregation, provide a protecting barrier against pathogens [34, 40-42], and increase water acquisition [2, 19, 26-27, 43-45]. Besides the structural and nutritional edges, AM fungi can facilitate crops deal with environmental stresses, thus enhancing plant growth by manufacturing metabolites such as amino acids, vitamins, phytohormones, and antioxidant enzymes and adjusting plant physiological status such as amino acid content, carbon dioxide exchange rate, and stomatal conductance [2, 7, 26, 44, 46-49]. AM fungal species (Glomus intraradices, Rhizophagus irregularis, Funneliformis mosseae and Rhizophagus fasciculatus) are accustomed to improving crop performance below salinity and drought stresses [2, 14, 50-55].
Trichoderma could be a filamentous fungus, opportunist, avirulent symbionts that are used as biopesticide, biofertilizer or fertility promoter to most crops in worldwide. Trichoderma species promotes the expansion of plants, yield, increase nutrient accessibility and limits the growth of plant pathogens. Trichoderma species are effective biofungicides, enzymatically degrading alternative fungi, manufacturing anti-microbial compounds that kill pathogenic fungi, and outcompeting pathogenic fungi for space and nutrients. Trichoderma grows on the surface of roots, wherever it provides malady management and enhances root growth and protects roots from certain physical stresses, permitting the roots to grow quicker. Trichoderma kills numerous major root rot fungi: Pythium, Rhizoctonia, and Fusarium. The process is named mycoparasitism. Trichoderma plays a very important role within the bioremediation of soil that are contaminated with pesticides and herbicides. Aspergillus, Penicillium, Piriformospora, Phoma, Rhizoctonia and many others PGPF could be used fertility supporter to most of the agricultural crops in global ecosystem.
Microbial Consortia (MC):
Collaborations between completely different PBMS and biological nitrogen fixing bacteria (BNFB) and host plants are often essential to keep up soil fertility (SF) and plant health (PH), significantly in low-input agriculture that depends on biological process instead of agrochemicals [56]. Combinations of various PBMS, as MC, may end up in improved overall plant performance. PGPB are shown to absolutely influence legume–rhizobia and plant–fungi interactions [7, 13, 57-59]. The combined use of PGPB and BNFB can improve root growth and plant resilience to environmental stresses, and scale back N losses [60]. MC of N (Azotobacter/Acetobacter), P (Pseudomonas) and K (Fraturia) helps in increasing the nutrients accessibility for a healthy growth, higher yield and provides protection to the crops from the pathogens. It’s well known that BNFB are often wont to ameliorate nodule formation in legumes once co-inoculated with rhizobia [7-8, 61] and enhance plant growth (PG) indirectly by optimizing the connection between host plants and AM fungi. Prasad [15] found that Glomus fasciculatum and Pseudomonas striata improved biomass growth and nutrient uptake of Azadirachta indica. Moreover, AM fungi also can keep company with legumes wherever rhizobia are present to increase grain yield and protein content [7, 9, 53-54].
Seed Coating with Beneficial Microorganisms (BMO):
SC is that the method of exogenous materials onto the surface of seeds with the aim of improving seed appearance, performances and handling characteristics such as seed weight and size and/or delivering active compounds like plant growth regulators (PGR), micronutrients, and microbial inoculants (MI) which can shield the seed against phytopathogens and increase establishment, germination and PG [14, 27, 62]. SC is applied by agricultural, horticultural and crop industries worldwide and has attained its place within the international market [62]. It’s used for applying colours and tracers (fluorescent dyes); protectants (pesticides); soil adjuvants (Soil hydrophilic materials and hydro-absorbers); compounds that stimulate germination, growth, and stress resistance (salicylic acid, gibberellin acid, and abscisic acid); macro and micronutrients, PBMS and BNFB inoculants [14, 16, 62-63]. Coating seeds with PBMS permits an explicit use of minor amounts of inoculum at the seed-soil interface [63], guaranteeing that the PBMS are without delay accessible at germination and early development plant stages, stimulating healthy, fast establishment and consequently increasing crop production [2, 64].
Types of Seed Coating:
Film Coating (FC):
FC may be a terribly skinny film layer around the outer surface of seed wall. The film coat materials consist of polymer, a plasticiser and colorant [65-66] increasing the weight of the seed by 2%-5% without changing the shape of the seed [67]. Film coated seeds are safe and increase growth and productivity of the crops.
Pelleting:
Pelleting may be a wet operation wherever the seeds enclosed with the filler material to obtain a uniform shape and therefore useful in exactness planting [68]. It can increase the weight of seed as much as 35% and conjointly helps within the overcome the stress conditions underneath low holding capability.
Encrusting:
An intermediate between FC and pelleting is termed as encrusting. It’s the same as FC, that sometimes don't amendment the shape of the seed, however added a bit weight to the seed. Associate encrusting agent is used that once absorbs the water, swells, split and releases the coating around the seed within the soil atmosphere.
Biological Seed Coatings (BSC):
The term BSC represents coating of beneficial microbes (BM) on seed surface. BSC is applied in two ways: (i) Pre coating of seeds, and (ii) On site, as a seed coating simply before sowing. the foremost widespread technique is on site technique primarily due to lower cost and short survival of BM however it's some major drawbacks as [1] Additional steps during sowing for a farmer [2] During field intermixture, there are some probabilities of decrease in germination percentage [3] Possibility of uneven coating on seed surface, [4] Higher doses are needed [5] Adhesion of microbes to seed is poor. Pre-coating of seeds has deserved to eliminate all or most of the above-mentioned difficulties. As farmer gets a ready to use products and is additional cost effective and eco-friendly than the soil vaccination and on-site coating, wherever farmer needs additional inoculums and obtain direct exposure to formulations which can cause activity hazards to them. This drawback is resolved by applying the ingredients (bacterial and fungal inoculants) directly onto the seed surface. McQuilken et al. [69] tested the efficacy of Pythium oligandrum against Pythium damping off of sugar beet. Pelleting was done Oospores of Pythium oligandrum and clay Falcate. Results showed that coating on seed was effectively controlled the damping off disease. The coating was done by adding the culture and seeds in polythene bag and then shakes it to provide a FC of the fungal culture on the seeds. The behaviour of biological differs at each stage from broth culture - coated seed surface – storage - field. To increase the survival, freeze drying and lyophilization process were introduced, however upon desiccation, quantity of viable microbial cells falls from the seeds. The above drawback is overcome using victimization the microencapsulation technique of microbes.
Microencapsulation:
Encapsulation of microbes with polymers is developed to enhance their shelf life on seed. This approach is extremely helpful for seed coating technology industries. Encapsulation is that the technique of generating a protecting shell around the microbes. It’s several benefits over the conventional formulations; encapsulation of microbes protects them from adverse outer environmental conditions, permits controlled release of cells to the surrounded environment [70] and conjointly helps in improving the viability of microorganisms. Microencapsulation is sometimes done by encapsulating the cells with surface coating materials like resins and plastics. However, microencapsulation has few drawbacks such as microencapsulated microbes are in less contact with the seed which can hinder bacteria to move through the soil towards plants and huge loss of inoculants on seed throughout microencapsulation preparation [31]. Mostly microencapsulation is finished with polymers. Numerous strategies are used for microencapsulation of bacterial cells: extrusion, spray drying, emulsion technique, solvent extraction, and thermal gelation, coacervation [71]. Various factors have an effect on the microencapsulation technique; like resistance and mechanical stability of coating material for a capsule. These factors are terribly crucial for storage purpose and agricultural applications.
Seed Coating Ingredients (SCI):
In SC, a binder consists of a polymer which can be natural or synthetic. The aim of using polymer in SC is due to their adhesive properties that ensures dust-free handling of seeds and make them smooth and foldable. Additionally, polymer coating provides a seed extra shell that protects it through direct exposure to unfavourable environmental conditions throughout storage. Once reviewing literature on polymers as binders/adhesives that are used in seed coating from the last two decades viz., Polymers- PVAs; Polyvinyl acetate copolymers (ethylene); Polyvinyl alcohols; Polyvinyl alcohol copolymers; Polyvinyl acrylates; Polyvinylpyrolidones; Vinylidene chloride Vinylidenechloride copolymers; Acrylic copolymers, Cellulose-ehylcelluloses, Methylcellulose, Hydroxymethlycellulose, Hydroxypropylcellulose, Hydroxyl methylpropylcelluloses, Dextrin’s, Maltodextrins, Polysaccharide and alternative binder are Fats, Oils, Proteins, Gum Arabic, Shellacs, Calcium lignosulfonates, Starch are used for this purpose.
Polymer properties (chemical and thermal) are analyzed by performing some typical tests which has density, durability, tensile strength, crystalline melting point, glass temperature. Polymers are divided into two broad classes: Hydrophobic and Hydrophilic. Several polymers are used in number of seed coating studies relying upon the most purpose of polymer coating like some polymers are wont to delay the germination or some to boost the germination rate. Hydrophobic polymers are used with an aim of developing a formulation which might defend the seed in soil until the condition for germination is poor [72-73]. Hydrophilic polymers are wont to fight against drought conditions. This polymer has high surface area and hydration-dehydration properties that prompted the development of slow unleash fertilizers [74]. Chachalis and Smith [75] reportable that the coating of soybean with hydrophobic polymer was improved the germination and seedling emergence. Bardin and Huang [76] conducted a study to examine the effectiveness of ten stickers/polymers to be used in seed coating for the management of plant disease caused by Pythium spp. Out of ten stickers , the foremost effective were polyvinyl alcohol, methyl cellulose, alginate and carrageenan. Gum-Biopolymers have conjointly been used as each adhesives and protectants from fungal diseases like pearl millet downy mildew in pearl millet seed coatings. Variety of trees as Acacia arabica, Moringaoleifera, Carcia papaya and Azadurachta indica are documented to provide gum exudates. These biopolymers are dry quickly, dissolve rapidly in water and don't inhibit germination. Coating of polymers are glorious to provide a protecting micro environments to seed natural coat however there are a number of the polymers are found compatible with chemical fungicide such as Captan, thiram, Goucho [77-78].
Fillers/Carriers:
Fillers are used for increasing the loading rate of the active ingredients (mostly in pelleting and encrusting) for following characters. a. The properties of the carrier materials of seed coating area should be porous, to permit air movement into the seed b. Coating must weaken or break down because it comes in contact with soil moisture c. It ought to be non-toxic. d. Able to apply on commercial bases. e. Size of particles ought to be specified it passes through 300 mesh sieve size. Stella and Sivasakthivelan [79] conducted a study by using completely different organic amendents such as sawdust, paddy, straw powder, wood charcoal, farmyard manure and poultry manure with lignite material for developing a formulation of Azospirilium lipoferum. Results showed that using these amendments within the formulation exaggerated the shelf-life of Azospirilium lipoferum up to 6 months with required population of bacterial cells. The most ordinarily used fillers in SC are as sugars fillers- dextrin, malt dextrin; cereal flours fillers- wheat flour, oat flour, barely flour; clays and inorganic solids- fillers- calcium bentonite, kaolin, china clay, talc, perlite, mica, vermiculite, silica’s, quartz powder, montmorillonite, attapulgite and different fillers- activated carbon, diatomaceous earth, calcium carbonate, wood floors are used for SC. All of these carriers are extensively utilized in preparation of bio formulations either as soil immunisation or treatment of seeds simply before sowing.
Seed Coating Market (SCM):
The uppermost players in seed treatments corporations are as Germains, Becker Underwood, Advanced Biological Marketing, Syngenta, Bayer Crop Science, Incotec, Kwizda, Landec- Ag, Novozymes, Preciseness Laboratories, and Brett Young etc. Cotton, Beans, Pea, Lentil, Soybean, Tomato, Wheat, Maize and Chickpea are highest revenue generating crops of the tropical and sub-tropical regions of the planet and is taken into account to be native to peninsular India. Soybean second biggest revenue generating segment with around 14% share in world industrial seed market. Only a few products are offered as seed coating materials like polymers and fungicide. Intrinsically presently no biological seed coating has been commercialised within the market as seed coating merchandise. Thus, there's lots of scope for developing the biological merchandise for SC.
Benefits for Biological Seed Coating:
SC is beneficial for increase plant growth and productivity as [1]. It’s provides protection to seed from the first day onward [2]. It’s extremely précised technique, wherever a really less quantity of total coating material is required as compare to soil immunisation and foliar spray applications. [3]. SC minimizes the occupational hazards that a farmer typically gets throughout foliar sprays and on-site coatings of seeds and conjointly minimizes the chance of accidental loss to the atmosphere. [4]. It’s conjointly provides uniform coverage to seeds that lacks in conventional coatings [5]. Seed coating are reducing agricultural machinery and its maintenance costs, saves farmers time, that successively, will increase a farmer’s financial gain [8]. Recent advances in seed coating technologies have resulted within the introduction of recent innovations: biological and nutrients which can facilitate in harmonizing nature further as agriculture (Figure1).
Challenges in Developing Formulation for Seed Coating with Combination of Fungicides, Polymers, Biological and Nutrients:
Numerous studies are conducted on BSC with totally different materials like polymer, carriers, fungicides, insecticide etc. however the studies remained confined to the laboratories solely. Till date, there's no formulation for SC with biological alone or in combinations with different seed coating materials, are accessible on the commercial scale. The foremost limiting step in commercializing the biological formulation (BF) for SC is shelf-life. Apart from shelf life, compatibility of biological with polymers and different ingredients of coating assurance of correct loading of precise number of cells on seed and stability are different vital characters if coatings ought to be applied as multilayer [80]. All these studies are still in experimental field as a result of most of the formulations developed are tested below artificial and controlled conditions and so established inadequate for field conditions [81]. Microbes conjointly need aeration to survive and stay alive until the coated reaches the soil. Thus, porosity of the polymer is additionally a vital factor in survivability of microbes and effectiveness of the coating. A perfect coating with microbes ought to have following characteristics; a. Fungicide ought to be compatible with microbes b. Polymer ought to have hygroscopic and moisture retention properties c. Coating ought to be skinny, water soluble and may not hamper the normal germination of seed. Following are the key points to take care of shelf-life of biological formulations (Figure 2)
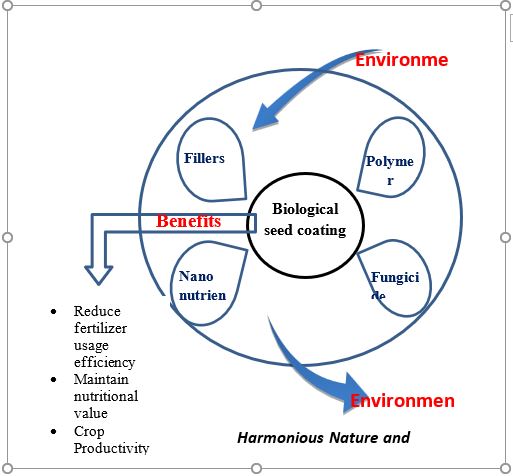
Figure 1: Diagrammatic Model presentation of benefits of biological seed coating
Mode of Action of Microbial Coated Seeds:
As the root system develops, the microbes grow with the roots extending the protection throughout the season. As results of this biological protection, an energetic root system is established by the plant, which frequently leads to additional uniform stands and larger yields [5, 13, 15, 82-84].
High Value Crops for Microbial Seed Coating:
There are various crops grown for food around the world. The paddy, vegetables, wheat, oil seed crops, chickpea, mung bean, soybean are among high inputs crops. Except for their economic value, these crops are majorly attacked by fungus Rhizoctonia solani and Pythium spp. that causes severe economic lose to farmers [85-86]. Because of these reasons, these crops are targeted by seed coating companies for treatments with biological fungicide and pesticide. Coating of soybean seeds with mycorrhiza, Trichoderma viride and Gliocladium virens spores has considerably inhibited growth of plant pathogenic fungi.

Figure 2: Schematic representation of check points to achieve successful loading of beneficial microbes on seeds and maintain long shelf-life
Symbiotic Action of Microbial Coated Seeds (MCS):
The beneficial microbes on in seed penetrate and colonize the plant roots and transmit filaments into the encircling soil. These filaments form a bridge that connects the plant roots with giant areas of soil (up to two hundred times larger than the root zone) and act as a "pipeline" to funnel nutrients to the plant. In return, the plant discharges compounds, through its roots, to stimulate fungal growth. The effectiveness of the fungi is additionally increased by soil microbes such as mycorrhizae and Bacillus spp. These beneficial microbes improve the root colonization and performance by manufacturing enzymes, hormones, vitamins, and alternative factors that promote the health and therefore the performance of the crops.
Role of MCS in Healthful Diet:
Pulses are sorts of legume (seeds that grow inside pods) include chickpeas (also referred to as garbanzo beans), lentils and dry peas. Microbial seed coated pulses increase the yield also as physical and chemical properties. Pulses provide macromolecule, dietary fiber, and lots of vitamins and minerals. They additionally contain “phytochemicals” (plant chemicals), which can cut back the risk of certain forms of cancer and alternative diseases.
Use of MSC Produce Pulses in Special Diets:
As results of their nutrients content and alternative properties, organic pulses can play a significant role in numerous special diets in human worldwide.
Gluten-free Diet (GFD):
Someone with celiac disease consumes gluten (a Protein macromolecule found in wheat and a few alternative cereal grains); an immune reaction is triggered within the small intestine, which may cause injury and poor absorption of nutrients. Organic pulses contain no gluten; thus, people with celiac disease can use organic chickpeas, lentils or peas as an ingredient in recipes.
Diabetic Diet (DD):
For people with polygenic disease, consuming organic lentils, peas and beans might facilitate with blooglucose management. Compared with another carbohydrate sources, pulses have a lower glycemic index. Some studies have shown that consuming organic pulses might result in additional stable blood glucose levels once meals.
Vegetarian Diet:
Pulses and vegetables are sensible sources of macromolecule, vitamins and minerals (especially iron and zinc), that makes them a wonderful food selection for vegetarians. They contain eight essential amino acids. Consuming lentils with rice provide the complete complement of amino acids required for growth and netter health. It is full fill by MSC seeds cultivated and products use for human health.
Weight Management Diet (WMD):
Although additional studies are required, intense pulses and vegetables might facilitate with weight management. For people trying to lose weight, pulses and some vegetables are high in fiber and macromolecule, low in fat and moderate in calories. One cup of cooked lentils or dry peas contains concerning 1/2 the daily fiber recommendation for adults. Foods higher in fiber content sometimes facilitate people feel “full” or satiated at mealtime. MSC is appropriate for use on cereals, millets, pulses, oilseeds, fiber crops, sugar crops, forage crops, plantation crops, vegetables, fruits, spices, flowers, medicinal crops, aromatic crops, orchards and ornamentals for large scale yield production.
Conclusions:
The objective of this manuscript is to focus on presently memorable regarding seed coating strategies that are developed in understanding the importance both in term of process and effects. BSC can provide a technique of harmonizing nature in agriculture. There are terribly little/no commercial formulations containing BM, fungicides, polymer and nutrients thus far. By incorporating biological in conventional pre-coating process, seed companies can provide farmers with a convenient ready-to-use product. BSC represent additional expense in material and process; however conjointly supply a spread of individual or combined benefits that overweigh the expense. Thus, keeping view the hidden constraints in BSC, there's an urgent need of developing a novel formulation with better shelf-life and reduces energy inputs. SC conjointly acts as a temperature switch and protecting coating by regulation the seed uptake of water, until the soil has warmed to a predetermined temperature. It conjointly makes room for together with all the specified ingredients such as inoculants, protestants, nutrients, herbicides, oxygen suppliers etc. It conjointly provides resistance against mechanical injury within the seed drill.
Conflict of Interest:
The author of this manuscript confirms that there is not any conflict of interest associated with the manuscript.