
NA Aliyev1* and ZN Aliyev2
1Azerbaijan State Advanced Training Institute for Doctors named by A.Aliyev, department of psychiatry and drug addiction, Baku, Azerbaijan
2Azerbaijan Medical University, department of psychiatry Baku, Azerbaijan
*Corresponding Author: NA Aliyev, Azerbaijan State Advanced Training Institute for Doctors named by A.Aliyev, department of psychiatry and drug addiction, Baku, Azerbaijan
Received: March 26, 2021
Accepted: March 31, 2021
Published: April 05, 2021
Citation: NA Aliyev, ZN Aliyev, “Valproate (depakine-chrono) in the acute treatment of outpatients with benzodiazepine addiction therapy during COVID-19 pandemic: Randomized, double-blind placebo-controlled study”, Aditum Journal of Clinical and Biomedical Research, 1(1); DOI: http;//doi.org/04.2021/1.1001.
Copyright: © 2021 NA Aliyev. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objective:
Non-medical use of prescription benzodiazepines, has been a long- established problem that has been associated with a large number of overdose deaths worldwide. Most recently, the concomitant use of benzodiazepines and opioids has been linked to a number of deaths that occurred in the recent opioid crisis, featuring in a rising number of fatalities and adverse events in North America and in Europe
Method:
Eighty patients (100 all men) whom we studied were under observation in Mental The method of randomization was given by lottery. Each patient was randomized to receive either depakine-chrono (50 patients) 500 mg three times per day for 6 weeks or matched placebo (50 patients) in a double-blind manner. A structured clinical interview, for DSM-5Axis I Disorder, Patient Edition, was used to diagnose benzodiazepine addiction disorder according to DSM-5.
Results:
All patients (50) treated with depakine-chrono treated participants responded by 12 weeks, versus two of the 50 placebo-treated participants (p<0.001). The most common and problematic side effect in the depakine-chrono group was dizziness.
Conclusions:
The authors believe this to be the first double-blind placebo-controlled randomization study to test the efficacy of a depakine-chrono in the management of benzodiazepine addiction disorder. They need to be replicated in a larger study group.
Introduction:
Non-medical use of prescription benzodiazepines has been a long- established problem that has been associated with a large number of overdose deaths worldwide. Most recently, the concomitant use of benzodiazepines and opioids has been linked to a number of deaths that occurred in the recent opioid crisis, featuring in a rising number of fatalities and adverse events in North America and in Europe. In recent years, several new psychoactive substances (NPS) belonging to the benzodiazepine class have also emerged on the market and are being sold under street names such as “legal benzodiazepines”, “designer benzodiazepines”, and “research chemicals”. The use of such NPS belonging to the benzodiazepine class and the non-medical use of pharmaceutical benzodiazepines pose a great threat to public health [1].
In early publications, we showed that COVID-19 contributes to a number of mental disorders in the pandemic. People face a myriad of stressors due to the COVID-19 crisis, including fear of contracting and infecting others, inadequate access to testing, disruption to routine health care, financial loss, suffering associated with social distancing and quarantine, and uncertainty about the duration of the pandemic. In this case, people resort to the uncontrolled use of benzodiazepines. Because benzodiazepines have immediate anxiolytic, anti-stress, anti-phobic, and other effects [2]. It is known that, with a separate uncontrolled use of benzodiazepines, it causes dependence on these groups of drugs. The FDA recently released evidence that benzodiazepine use for more than two weeks causes substance abuse.
Thirty-three benzodiazepines were included in Schedule IV of the 1971 United Nations Convention on Psychotropic Substances in 1984. Midazolam (1990) and brotizolam (1995) were subsequently added to the Schedule. In 1995, flunitrazepam (CAS 1622-62-4) was transferred from Schedule IV to Schedule III because the International Narcotics Control Board (INCB) stated that it was one of the most misused benzodiazepines and because of its frequent diversion into the illicit market. Phenazepam (fenazepam) (CAS 51753-57-2), which is used in medical practice in some countries outside of the European Union, is not scheduled in the 1971 United Nations Convention on Psychotropic Substances. The INCB reported that in 2006, total global licit production of benzodiazepines amounted to at least 180 metric tonnes, 56 tonnes of which was diazepam. Italy (32 %), India (19 %), China (11 %) and Germany (10 %) were the leading manufacturers between 1997 and 2006 [3].
The main goal of this study was to find a new drug, and to conduct a double-blind, randomized, double placebo-controlled trial for the treatment of benzodiazepine dependence.
Materials and methods:
This was a double-blind, placebo-controlled trial for patients diagnosed with DSM-5 for benzodiazepine addiction disorder. The patients gave their informed, written consent to participate. In accordance with the Helsinki Declaration of the World Medical Association “Recommendations for doctors engaged in biomedical research involving people”, adopted by the 18th World Medical Assembly (Finland, 1964, revised in Japan in 1975, Italy - 1983, Hong Kong - 1989, the South African Republic - 1996, Edinburgh - 2000); The Constitution of the Republic of Azerbaijan, the Law “On Psychiatric Assistance” (adopted on 12.06.2001, with amendments and additions - 11.11.2011, Decisions of the Cabinet of Ministers of the Republic of Azerbaijan No. 83, dated April 30, 2010 “On Approval of the Rules for Conducting Scientific, Preclinical and Clinical studies of medicines” are established. The conditions of the conducted researches corresponded to the generally accepted norms of morality, the requirements of ethical and legal norms, as well as the rights, interests and personal dignity of the participants of the studies were observed.
The study periods: from March 1, 2020 to March 1, 2021. Patients were observed at the Mental Health Center of the Ministry of Health of the Republic of Azerbaijan. This was a double-blind, placebo-controlled trial for patients diagnosed with DSM-5 for benzodiazepine addiction disorder. A structured clinical interview, for DSM-5Axis I Disorder, Patient Edition, was used to diagnose benzodiazepine addiction disorder according to DSM-5. The condition of the patients was assessed as moderate: presence of 4-5 symptoms (304.10 (F13.20) [4].
Hundred patients (100 all men) whom we studied were under observation in Mental Health Center of the Ministry of Health of the Republic of Azerbaijan. The method of randomization was given by lottery. Each patient was randomized to re¬ceive either depakine-chrono (50 patients) 500 mg three times per day for 12 weeks or matched placebo (50 patients) in a double-blind manner. We were administered depakine-chrono three times daily. But some authors recommended [5] that depakine-chrono must be administered once daily. Our suggestion the adminis¬tered of depakine-chrono three times daily were based on the following data: (1) it is known that the mean time to reach plasma concentration (? Max) for controlled-release tables of depakine (500 mg — 2000 mg) administered as single doses to health volunteers ranged from 5 to 10 h [6,7], (2) our preliminary unpublished study showed that patients whom adminis¬tered depakine-chrono once daily dose strategy has more side effects, were advisable and not really necessary. It is nec¬essary specially to mark that patients often to complain steady high somnolence; and (3) our patients with desire and virtues a receiver of depakine-chrono three times daily. On this base we administered depakine-chrono three times in day.
Eligible participants were required to be between 18 and 65 years of age.
Analysis of response refers to the last observation carried forward for all subjects who had valuables efficacy at baseline and with treatment. The responder analysis was conducted by using the chi-square (x2) and analysis of variance (ANOVA) according to Glantz [8].
Results:
Characteristics of the patients randomly assigned to the two treatments are shown in table 1
|
Characteristic |
Depakine-chrono n =50 |
Placebo n = 50 |
|
|
Mean SD |
Mean SD |
|
Age (years) |
37.0 ± 9.2 |
38.4 ± 9.8 |
|
Duration of illness in month |
6,2±0,5 |
5,9±0,6 |
|
Education: — primary school — secondary school |
28 (56%) 22 (44%) |
30 (60%) 20 (40%) |
|
Marital status: — never married — married — divorced or separated |
10 (20%) 30 (60%) 10 (20) |
10 (20%) 28 (56) 12 (24) |
|
Employment status — unemployed — employed |
18 (36%) 32 (64%) |
22 (44%) 28 (56) |
Note: differences between groups are not significant
Table 1: Sociodemographic characteristics of patients with rapid cycle bipolar disorder
Statistical differences between the two groups are not significantly. The results of treatment are shown in Table 2. Response was defined as a 100% reduction in the symptoms of benzodiazepine addiction disorder.
The responder was conducted by X2 demonstrated superior for than for placebo (Table2). Depakine-chrono was generally well tolerated by the patients in the study, although more Depakine-chrono patients (n = 2) discontinued the study early because of adverse events. The two common side effects leading to discontinuation in the depakine-chrono group were allergic reaction and drowsiness. Sweating a frequent complaint during placebo treatment occurred in two of 50 men. There were not unexpected or serious adverse events. From depakine-chrono group patients improvement observed in 48 while in the placebo group, improvement was noted in only 2 patients.
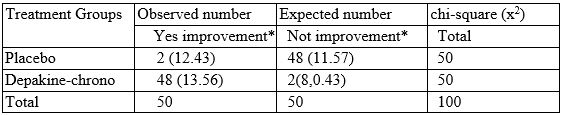
Note: expected numbers indication in the brackets %2 = 22.68, df = 1, p< 0.001.
Table 2: The results of the square analysis.
Discussion:
Problems of pharmacological treatment of abstinence, dependence and withdrawal of benzodiazepines are devoted to a number of works [9]. Among them, we especially want to note the work of Fluyau with colleagues [10]. They offer several drugs for the pharmacological treatment of withdrawal, dependence and withdrawal of benzodiazepines: propranolol, clonidine, baclofen, lamotrigine, trazodone, carbamazepine, gabapentin, pregabalin and valproic acid and others. Note that most of these medications are anticonvulsants. Valproic acid (VPA) is an anticonvulsant drug used in psychiatry as a mood stabilizer in patients with bipolar disorder. VPA is a sedative, possibly due to increased neurotransmission of GABA receptors. Several studies have shown that VPA selectively accumulates GABA in the brain at the terminals of the neurotransmitter GABA.
Although the mechanism of action of valproate is not fully understood, traditionally, its anticonvulsant effect has been attributed to the blockade of voltage-gated sodium channels and increased brain levels of gamma-aminobutyric acid (GABA). Divalproex is converted to valproic acid in the stomach. Scientists do not know the mechanism of action of valproic acid. The most popular theory is that valproic acid exerts its effects by increasing the concentration of gamma-aminobutyric acid (GABA) in the brain. Benzodiazepines cause anxiolysis by acting on the γ-aminobutyric acid (GABA) -benzodiazepine receptor complex. GABA is synthesized from glutamic acid, the most abundant free amino acid in the CNS. Like serotonin, norepinephrine, and dopamine neurons, the presynaptic GABA neuron has a recirculation pump that transports GABA from the synapse to be maintained or destroyed by GABA transaminases. GABA has three target receptors: GABAA, GABAB and GABAC. The chloride ion channel is controlled by GABAA.
Four different pharmacological properties have been described for the benzodiazepine receptor: anxiolytic, hypnotic, anticonvulsant, and muscle relaxant effects. GABAA receptors with Α2 (and / or α3) subunits mediate anxiolytic effects, while GABAA receptors with α1 subunit mediate sedative-hypnotic actions. Most benzodiazepines interact with both receptor subtypes. Typically, when GABA occupies the GABAA receptor area, the opening and closing of the chloride channel occurs more frequently, and this effect is inhibitory. At the same time, if a benzodiazepine binds to a nearby benzodiazepine receptor, the GABAA receptor is allosterically modulated and has a greater effect on the GABA chloride channel and permeability (greater opening and closing frequency). Although GABA acts alone on the GABA receptor, the effect of GABA is stronger in the presence of benzodiazepines. In the absence of GABA, benzodiazepines cannot self-affect the chloride channel [11].
Overall, it cannot be denied that we are seeing more overdose-related deaths, addiction problems, and suicides [12, 13, 14].
A mental health crisis is expected due to the COVID-19 pandemic, and we are likely to see an even greater rise in anxiety and insomnia. Benzodiazepines may be tempting at this time due to their “quick fix” nature, but we know that this “quick fix” prevents patients from developing coping skills to deal with stressors. Patients become addicted to drugs, and therefore their long-term use becomes an addiction problem
As shown by the results of our early research, the COVID-19 pandemic has led to a significant increase in mental illness and exacerbated existing mental disorders [2]. Anxiety a feeling of worry, nervousness, or unease, typically about an imminent event or something with an uncertain outcome. This unique combination of emotional responses caused by COVID-19 - what has come to be known as COVID stress syndrome [15] - can be considered a significant contributor to the rise in the incidence of substance use in the general population. In addition to the unique consequences of interoceptive anxiety of BIS activation, the presence of substances of abuse makes this very likely and poses a serious public health problem. During this pandemic, shops distributing alcohol were considered important businesses and, combined with job loss, increased time at home and isolation, increased the likelihood of their use. The link between xenophobia and substance use is less clear, largely due to a lack of specific research. However, it is not surprising that xenophobia is associated with substance abuse, given the economic anxiety and fears of social displacement that contribute to its occurrence.
The European Monitoring Center for Drugs and Addiction [16] in Europe and the National Institute for Drug Abuse [17] in the United States were the first to sound the alarm, expressing concern about the vulnerability of people with substance use disorders to COVID-19. , especially due to the effects of opiates (eg heroin), synthetic opioids and methamphetamine on the respiratory system.
On September 23, 2020, the Food and Drug Administration (FDA) announced the need to update the Boxed Warning - the most visible one used, with the introduction of risk indications in the labeling of all drugs in this class abuse, misuse, physical dependence and withdrawal reactions to improve the safety of their use. In 2019, public pharmacies in the United States dispensed approximately 92 million prescriptions for benzodiazepines, of which the most common were alprazolam (38%), clonazepam (24%) and lorazepam (20%). In 2018, about 50% of patients receiving oral benzodiazepines used them for 2 months or longer, while most drugs in this group are recommended for weeks or months. Benzodiazepines are used in the treatment of generalized anxiety disorder, insomnia, seizures, social phobia, and panic disorder. They are also used as a premedication before certain medical procedures. The dose, frequency and duration of treatment will vary depending on the patient, the specific benzodiazepine prescribed, and the indication. Physical dependence can occur with chronic benzodiazepine use over several days or weeks. Patients who have been taking benzodiazepine for weeks or months may experience withdrawal signs and symptoms with abrupt discontinuation or rapid dose reduction, including life-threatening seizures. In addition to requiring that packaging warnings be updated, the FDA requires appropriate changes to the Warnings and Precautions, Abuse and Dependence, Patient Counseling Information, and existing patient and caregiver guidelines. [Based on materials www.fda.gov]
The COVID-19 pandemic has transformed the face of psychiatry over a very short time period. Given the detrimental impact of the pandemic on mental health and the economy, more difficult days are ahead for psychiatry. The rising public health burden of mental illnesses will inevitably exceed the capacity of psychiatric services in the United States and worldwide. The pandemic has also profoundly affected psychiatric research due to safety concerns and containment efforts. Intermediate and long-term ramifications may even be more serious. In addition to the effects of the economic downturn on available research funding, existing research tools and protocols may not meet the emerging needs in the post-COVID-19 era. This paper discusses potential trends and challenges that psychiatric practice and research may encounter in this period from the viewpoint of workers in the field. We outline some measures that clinicians and researchers can implement to adapt to the emerging changes in psychiatry and to mitigate the forthcoming effects of the crisis [18].
The COVID-19 pandemic has created a sense of danger, uncertainty, and loss of control in populations worldwide, placing mental health discussions high on the public’s agenda. Disasters traumatize societies, typically in a time-limited event with destructive outcomes that hit one community and require others to help. The pandemic is unusual because the world faces a danger with an unknown end date. Communities that support each other in normal times are now competing for scarce resources to cope with their own crises. The impact of this crisis on individuals and societies is compounded by the experience of facing danger without help. If the pandemic lasts for an extended period, as projected by some models [19, 20] psychiatric practice and the place of psychiatry in medicine are likely to undergo lasting changes. Here, we will (i) identify potential trends and challenges that psychiatric practice and research may encounter during this period, (ii) will suggest concrete measures that clinicians and researchers can take to mitigate the effects of this crisis at individual and institutional levels.
To our knowledge, this is the first report of a randomized, double-blind, placebo-controlled study of a valproate (depakine-chrono) in the acute Treatment of outpatients with benzodiazepine addiction disorders therapy during COVID-19 pandemic. Our data suggest that Valproate (depakine-chrono) is efficacious in the management of benzodiazepine addiction disorders therapy during COVID-19 pandemic, as the participants had a clinically and statistically significant improvement in addiction symptoms over 12 weeks of treatment.
Evidence exists, to a greater or lesser extent, that all of these agents posses anxiolytic properties, as would be expected by their mechanisms of action [21]. Valproic acid is a sample branched chain fatty acid that was originally developed for the treatment of epilepsy. In addition to its anticonvulsant activity, valproic acid has demonstrated anxiolytic, mood-stabilizing, antimigraine and antinociceptive effects and has been evaluated in the management of various other disorders, particularly psychiatric conditions. These activities appear to be mediated, at least in part, by its effects on GABA — mediated neurotransmission. Valproic acid increases CNS concentrations of GABA, possibly by increasing its synthesis and/or inhibiting its catabolism. Valproic acid has also been reported to decrease neurotransmission by the excitatory amino acids (-hydroxybutryc, aspatic and glutamic acids), to inhibit cell firing induced by Af-methyl-D-aspartate, and to exert a direct neuronal membrane depressant effectf via modulation of sodium and potassium conductance. Val-proic acid is generally well tolerated, does not induce hepatic drug metabolism and has a low propensity for interactions with psychotropic agents. However, as has been observed with several other antiepileptic drugs, it is teratogenic and can cause elevated hepatic enzyme levels and rare, fatal hepatotoxicity. Weight gain and alopecia are relatively common [22].
An experimental and clinico-pharmacological study of sodium valproate, a GABA-ergic drug, was conducted to elucidate the role of gamma-aminobytric acid in the mechanisms responsible for affective disturbances. A tranquilizing effect of the valproate, comparable with the action of diazepam, was established in a conflict situation model in experimental animals. It was established that this action was accompanied by no manifestations of miore-lexation, ataxia or somnolence characteristic of tranquilizers of the benzodiazepine series. The presence of tranquilizing properties in the GABA-ergic drug sodium valproate confirms the suggestion that a certain relationship exists between the GABA system and anxiety [23].
Valproic acid is a sample branched chain fatty acid that was originally developed for the treatment of epilepsy. In addition to its anticonvulsant activity, valproic acid has demonstrated anxiolytic, mood-stabilizing, antimigraine and antinociceptive effects and has been evaluated in the management of various other disorders, particularly psychiatric conditions. These activities appear to be mediated, at least in part, by its effects on GABA — mediated neurotransmission. Valproic acid increases CNS concentrations of GABA, possibly by increasing its synthesis and/or inhibiting its catabolism. Valproic acid has also been reported to decrease neurotransmission by the excitatory amino acids (-hydroxybutryc, aspatic and glutamic acids), to inhibit cell firing induced by Af-methyl-D-aspartate, and to exert a direct neuronal membrane depressant effectf via modulation of sodium and potassium conductance. Val-proic acid is generally well tolerated, does not induce hepatic drug metabolism and has a low propensity for interactions with psychotropic agents. However, as has been observed with several other antiepileptic drugs, it is teratogenic and can cause elevated hepatic enzyme levels and rare, fatal hepatotoxicity. Weight gain and alopecia are relatively common [24].
There has been a staggering increase in the number of benzodiazepine prescriptions in the last couple of decades in the USA. According to a serial cross-sectional study (January 1, 2003, through December 31, 2015) benzodiazepine prescriptions in outpatient settings increased substantially from 3.8% to 7.4% of visits. Psychiatrists prescribing benzodiazepines have been stable (29.6% vs 30.2%) but primary care physicians have increased (3.6% vs 7.5%)5. Primary care accounted for about half of all benzodiazepine prescriptions. This pattern of the rise of benzodiazepine prescriptions is very concerning amid the opioid epidemic and the COVID-19 pandemic. As per NIDA, opioid-related overdose deaths increased from 3,442 in 1999 to 17,029 in 2017 but the number of deaths dropped to 14,975 from 2017-2018 in the USA. Deaths due to benzodiazepine overdose increased from 1,135 in 1999 to 11,537 in 2017 but it declined to 10,724 from 2017 and 2018 in the USA 2. As per the New York Times, this decrease was probably due to tightening of the opioid prescriptions [25].
In any event, pending a further understanding of valproate's mechanisms of action, the present data suggest that this drug is a useful new agent for the treatment of benzodiazepine addiction disorders. It will be important to explore further effects of valproate in other addiction disorders.
Recommendation:
Limitation of the study: 1. The study must be carried out on a large number of populations; 2. The study must be carried out separately for women and men; 3. Study intermission duration of more than five years. Two limitations should be noted. First, our small study group and we recommend that these results be replicated in a larger group so that effect sizes can be more precisely estimated. Second, it is necessary study of possibility generalizability these data to women. Notwithstanding these limitations, this study suggests that, depakine-chrono are efficacious and well tolerated in the treatment of benzodiazepine addiction disorders.
In any event, pending a further understanding of valproate's mechanisms of action, the present data suggest that this drug is a useful new agent for the treatment of benzodiazepine addiction disorders. It will be important to explore further effects of valproate in other addiction disorders.
The authors declare that the article is submitted on behalf of all authors. None of the material in the article has been published previously in any form and none of the material is currently under consideration for publication elsewhere other than noted in the cover letter to the editor. Authors declare no financial and personal relationship with other people or organizations that could inappropriately influence this work. All authors contributed to and have approved the final article. The authors declare no conflicts of interest. No sponsor provided funding for this study. Mental Health Center of the Ministry of Health of the Republic of Azerbaijan provided the outpatient unit, the material for clinical and neuro-psychological assessments, and electronic resources.