Shamma AlZaabi1, Abdul-Kader Souid2*
Department of Pediatrics, College of Medicine and Health Sciences - UAE University, Alain City, United Arab Emirates
*Corresponding author: Abdul-Kader Souid, Department of Pediatrics, College of Medicine and Health Sciences - UAE University, Alain City, United Arab Emirates
Received: March 14, 2021
Accepted: March 25, 2021
Published: March 30, 2021
Citation: Shamma AlZaabi, Abdul-Kader Souid, “Systemic inflammation in a boy who had received the Verorab rabies vaccine: Case report”, J Pediatrics and Child Health Issues, 2(1); DOI: http;//doi.org/03.2021/1.1015.
Copyright: © 2021 Abdul-Kader Souid. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
The inactivated Vero-cell (an African green monkey kidney cell line) rabies vaccines, such as Verorab have been associated with serum sickness. Yet, diagnostic investigations that relate this systemic inflammation to the vaccine are not readily available. Under reporting ‘vaccine-related systemic inflammation’ imposes a significant risk to children, who may be erroneously labeled as having a life-long rheumatoid or immunologic disorder. Moreover, in our region, the prevalence, natural history, and variables associated with this adverse event of rabies vaccines are unknown. We report here a 3-year-old previously healthy boy who developed high-fever, itching/salmon-pink skin rash, polyarthritis, and severe inflammation about three months after receiving Verorab. Serum sickness, systemic juvenile rheumatoid arthritis, and macrophage activation syndrome were considered as probable etiologies of his condition. This example illustrates the importance of understanding the clinical complexities associated with Vero cell vaccines and the needs for improved reporting of their potential adverse events.
Introduction
The World Health Organization (WHO) recommends immunization against rabies for children living or traveling to areas of high exposure rate, as well as those playing with animals (e.g., dogs, bats, raccoons, skunks, and foxes) who may not report bites [1]. This vaccination, however, is more often started after a justified exposure [2]. For this purpose, the purified (inactivated) Vero cell rabies vaccine (e.g., the French purified Vero cell vaccine, Verorab) has been the product of choice in our region; this preparation contains human albumin [3]. Possible adverse events of Verorab include allergic reactions, such as skin rashes, intense itching (urticaria, pruritus), swelling, respiratory symptoms, anaphylactic reaction, and serum sickness [4].
This case report describes potential adverse events of Verorab, which overlap with systemic inflammatory conditions, such as juvenile idiopathic arthritis (JIA), macrophage activation syndrome (MAS), hemophagocytic lymphohistiocytosis (HLH), and Kawasaki disease. This issue is convoluted by the lack of readily available tests that could detect “Verorab-associated delayed serum sickness”. Furthermore, health-care providers should adhere to “label use” of the rabies vaccines to minimize an unnecessary exposure to these preparations. Genetic predisposition to adverse reactions of the cell culture-derived vaccines, such as Verorab needs to be considered and investigated [4].
Case Presentation
Informed consent to report this child’s findings and publish his figures was obtained from the mother. This previously healthy 3½-year-old boy (weight, ~14 kg) had a bite from his vaccinated house dog, which resulted in a superficial scratch on his wrist without a puncture wound, swelling, erythema, or bleeding. The WHO guidelines and categories of ‘contacts with suspect rabid animal’ are based on nature of the contact and seriousness of the wound (see Verorab insert). Although he was not bitten by a rabid dog, his exposure (‘minor scratch or abrasion without bleeding’) was considered Category II, which requires “wound washing and immediate vaccination”. He received Verorab (intramuscular injections of 2.5 units in 0.5 mL) on Days 0, 3, 14, and 31. On Day 180, he had sudden-onset of continual fever and intense itchy rash on his trunk and extremities. Two weeks later, he developed joint pain and refused to walk. His physical examination (on Day 235) was remarkable for being irritable, but consolable. He had salmon-pink raised patches and eczema-like rash with scratching marks (Fig. 1).
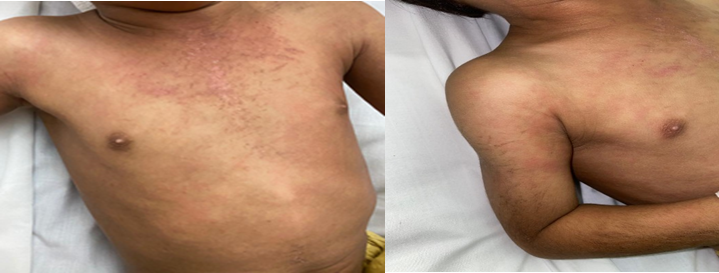
Fig. 1. Skin manifestations on Day 235.
He walked with difficulties due to pain. There was no swelling or redness over his joints, and no lymphadenopathy or hepatosplenomegaly. Eye examination by slit lamp showed normal findings. The course of his temperature is shown in Fig. 2. His C-reactive protein (CRP) and serum ferritin levels are shown in Fig. 3.
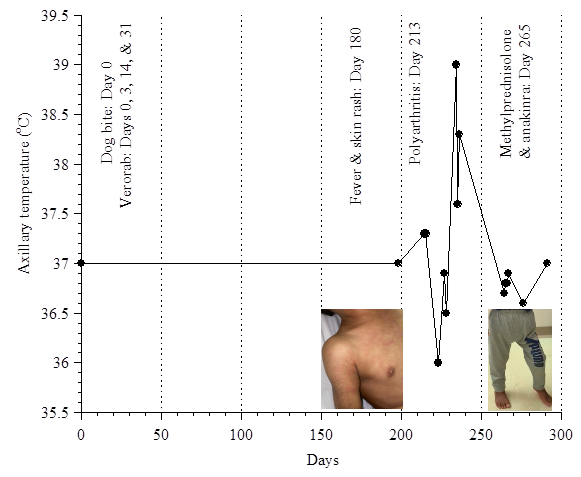
Fig. 2. The course of his temperature.
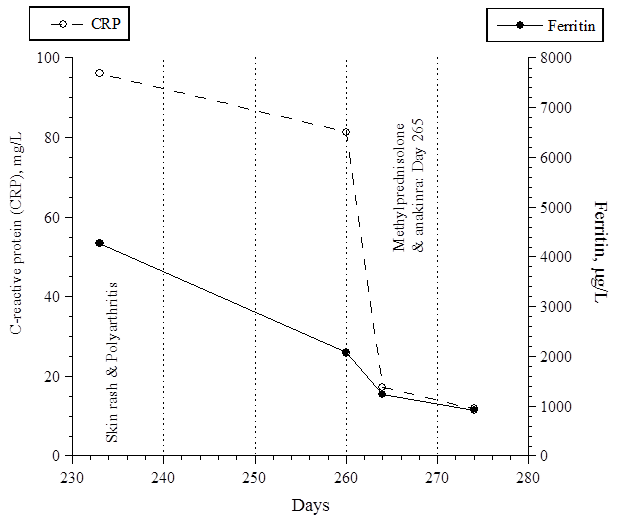
Fig. 3. His CRP and ferritin levels.
His serum triglyceride peaked on Day 264 at 1.45 mmol/L (reference range, 0.25 to 0.85) and nadired on Day 273 at 0.69 mmol/L. Complete blood counts showed progressive microcytic anemia with thrombocytosis, consistent with his systemic inflammation. On Day 235, his serum albumin was 24 g/L (reference range, 35 to 52). His urinalysis, serum electrolytes, creatinine, alanine aminotransferase, bilirubin, coagulation profile, and radiographies of the hip and knees were normal.
Discussion
Adverse events that could result from the administration of a rabies vaccine to children impose serious diagnostic and management challenges. Thus, the possibility of contracting the deadly rabies disease must be carefully weighed against the potential ‘vaccine-associated systemic inflammation’ [2]. The parents must be aware of these potential symptoms, especially with respect to inflammatory conditions such as JIA (requires excluding other potential entities) and MAS. Moreover, ‘rabies vaccine-associated systemic inflammation’ should be included in the differential diagnosis of these overlapping disorders.
It is also important to know that literatures on the potential adverse events of Verorab are lacking. The prevalence of an allergic reaction after booster doses of human diploid cell vaccine Imovax® is about 6% [4]. Systemic symptoms (e.g., headache, nausea, abdominal pain, muscle aches, and dizziness) occur with a frequency of about 20%. Type 3 hypersensitivity (IgG/ IgM-mediated, typically between day 2 and day 21 after booster doses) reactions, on the other hand, has been observed in about 10% of the recipients [4-5]. These reactions have been attributed to the vaccine ingredients, such as β-propiolactone and albumin [5]. The diagnosis is mainly clinical. Since JIA is a disease of exclusion, other potential etiologies must be considered, (e.g., recent rabies vaccination) prior to initiation of treatment. Genetic susceptibility is a key factor that affects vaccine-induced inflammation [6]. Future studies are needed to investigate this issue.
Conclusion: Vaccine-associated systemic inflammation should be included in the differential diagnosis of conditions, such as JIA and MAS. Pediatricians should be mindful of the "label use" and "off-label use" of anti-rabies vaccines and weigh the risk and benefits of their administration. Future studies are needed to determine the adverse events of rabies vaccines and their prevalence in our community.