Ann Q. Tran *1,2, Marissa K. Shoji1, Alexandra Levitt 1, Wendy W. Lee 1
1Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami, Miami, FL
2Department of Ophthalmology, Manhattan Eye and Ear Throat Hospital, Northwell Health, New York, NY
*Corresponding Author: Ann Q. Tran, Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami, Miami, FL.
Received: March 22, 2021
Accepted: March 25, 2021
Published: March 31, 2021
Citation: Ann Q. Tran, Vascular Endothelial Growth Factor Receptor Expression in Orbital Cavernous Malformations and Lymphatic Malformations, Ophthalmology and Vision Care, 1(1); DOI: http;//doi.org/03.2021/1.1003.
Copyright: © 2021 Ann Q. Tran. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly Cited.
Purpose:
To investigate the presence of vascular endothelial growth factor receptors (VEGFR) in orbital cavernous malformations and lymphatic malformations to further understand the feasibility of anti-VEGF treatment.
Methods:
This study was a single-center retrospective chart review performed at the Bascom Palmer Eye Institute of patients who underwent surgical excision of orbital cavernous malformations and lymphangiomas from 2000 – 2017. Immunohistochemical staining of these lesions for VEGFR1 and VEGFR2 expression was performed.
Results:
A total of 25 patients were identified with cavernous malformations (n=15) and lymphatic malformations (n=10). Ten specimens (7 cavernous malformations, 3 lymphatic malformations) underwent further immunohistochemical analysis. Six of 7 cavernous malformations and one of 3 lymphatic malformations stained positive for VEGFR1 and VEGFR2.
Conclusions:
Both cavernous malformations and lymphatic malformations appear to express VEGFR with varying frequency. Additional studies are needed to better characterize the pathogenesis of these lesions, nature of VEGFR expression, and potential efficacy of anti-VEGF treatment.
Introduction:
Cavernous and lymphatic malformations comprise a relatively high percentage of orbital lesions (Shields et al. 2004). Patients with these types of vascular malformations may clinically present with pain, edema, proptosis, restriction of eye movement, optic nerve compression, and amblyopia (Rootman et al. 2014 & Shoji et al. 2020). Although these lesions are not malignant, they have potentially serious sequelae, including mass effect on the globe and optic nerve, infection, and bleeding (Rootman et al. 2014, Elluru et al. 2014, Wiegand S et al. 2013, Woo et al. 2017).
Treatment of orbital vascular malformations can be challenging due to their location and risk of recurrence. In general, management is individualized based on the lesion type and location. Current treatment modalities include excisional or debulking surgery, drainage, sclerotherapy, and systemic therapy such as sirolimus (Shoji et al. 2020, Harris 1999, Wiegand et al. 2011, Macintosh et al. 2014, Cheng et al. 2015). Understanding the underlying pathophysiology may be beneficial to development of alternative treatment modalities.
Vascular endothelial growth factors (VEGF) are critical regulators of vascular and lymphatic function. They may be involved in the pathogenesis of these lesions and potentially offer a theoretical therapeutic target. Previous studies have examined the presence of VEGF receptors (VEGFR) in orbital tumors; however, these studies are small, and their results varied (Rootman et al. 2014, Nagasaka et al. 2007, Atchison et al. 2016). Therefore, this study aims to further characterize VEGFR expression in orbital cavernous and lymphatic malformations.
Methods:
A retrospective, single-center study was conducted at the Bascom Palmer Eye Institute. The research protocol was approved by the Institutional Review Board, adhered to the tenets set forth by the Declaration of Helsinki, and was Health Insurance Portability and Accountability Act compliant. A total of 25 patients who underwent complete surgical excision of orbital cavernous malformations and debulking of lymphatic malformations from 2000 to 2017 were included. Demographic, lesion, surgical, and pathologic specimen data were collected. An ocular pathologist reviewed all images.
Ten cases underwent immunohistochemical analysis. Surgical specimens were sent to the Florida Lions Ocular Pathology Laboratory. Formalin-fixed paraffin-embedded specimens were prepared. Immunohistochemistry was performed with antibodies for VEGF Receptor 1 (1:200; Abcam Cat# ab32152, RRID: AB_778798) and VEGF Receptor 2 (1:200; Cell Signaling Technology Cat# 9698, RRID: AB_11178792). Heat-mediated antigen retrieval with fresh sodium citrate buffer was performed followed by standard immunohistochemistry protocol. Images were taken with the Evos FL Auto Imaging System (Thermo Fisher Scientific, Waltham, MA, USA).
Results:
A total of 25 different patients were included in this study. 15 patients had cavernous malformations, (mean age 49.6±11.7 years old, 53% male). Patients presented with proptosis (66%), pain (26%), diplopia (13%), and compressive optic neuropathy (33%). Most lesions were intraconal (73%) and ovoid in shape with a uniform presentation. There was one case of bony erosion and once case of the lesion embedded within the temporalis muscle. All patients underwent complete surgical excision. The most common surgical approach based on the lesion location included a lateral orbitotomy approached from the lid crease (47%) lateral orbitotomy with bone flap (13%), swinging eyelid approach (20%), transcaruncular approach (13%) or subciliary approach (7%). Removal with cryotherapy was used in one case. No recurrences were noted after surgical excision. 7 specimens underwent immunohistochemical staining for VEGFR1 (6/7 positive)andVEGFR2(6/7positive,Table1,Figure1A-C).
|
Specimen number |
Specimen type |
VEGFR1 |
VEGFR2 |
|
1 |
Cavernous malformation |
+ |
+ |
|
2 |
Cavernous malformation |
+ |
+ |
|
3 |
Cavernous malformation |
+ |
+ |
|
4 |
Cavernous malformation |
- |
- |
|
5 |
Cavernous malformation |
+ |
+ |
|
6 |
Cavernous malformation |
+ |
+ |
|
7 |
Cavernous malformation |
+ |
+ |
|
8 |
Lymphatic malformation |
- |
+ |
|
9 |
Lymphatic malformation |
- |
- |
|
10 |
Lymphatic malformation |
+ |
- |
Abbreviations: + = positive, - = negative, VEGFR1: vascular endothelial growth factor receptor 1, VEGFR2: vascular endothelial growth factor receptor 2
Table 1: Immunohistochemical Staining of Specimens.
A total of 10 patients with lymphatic malformations were identified (mean age 26.1±24.8 years old, 30% male). The lesions were located intraconal (40%) and involved the eyelid (50%). Two patients had prior hemorrhagic episodes. The patients presented with proptosis (60%), diplopia (40%), and pain (20%). One patient had symptoms of compressive optic neuropathy. One patient had enlargement with Valsalva maneuver. All patients underwent orbitotomy with debulking. Five patients had additional recurrences of symptoms after initial debulking prompting additional surgery (30%), injection of a sclerosing agent with interventional radiology (20%), trial of sildenafil (10%) and trial of steroid injection (10%). A total of 3 specimens underwent immunohistochemical staining for VEGFR1 (1/3 positive) and VEGFR2 (1/3 positive, Table 1, Figure (D – E):
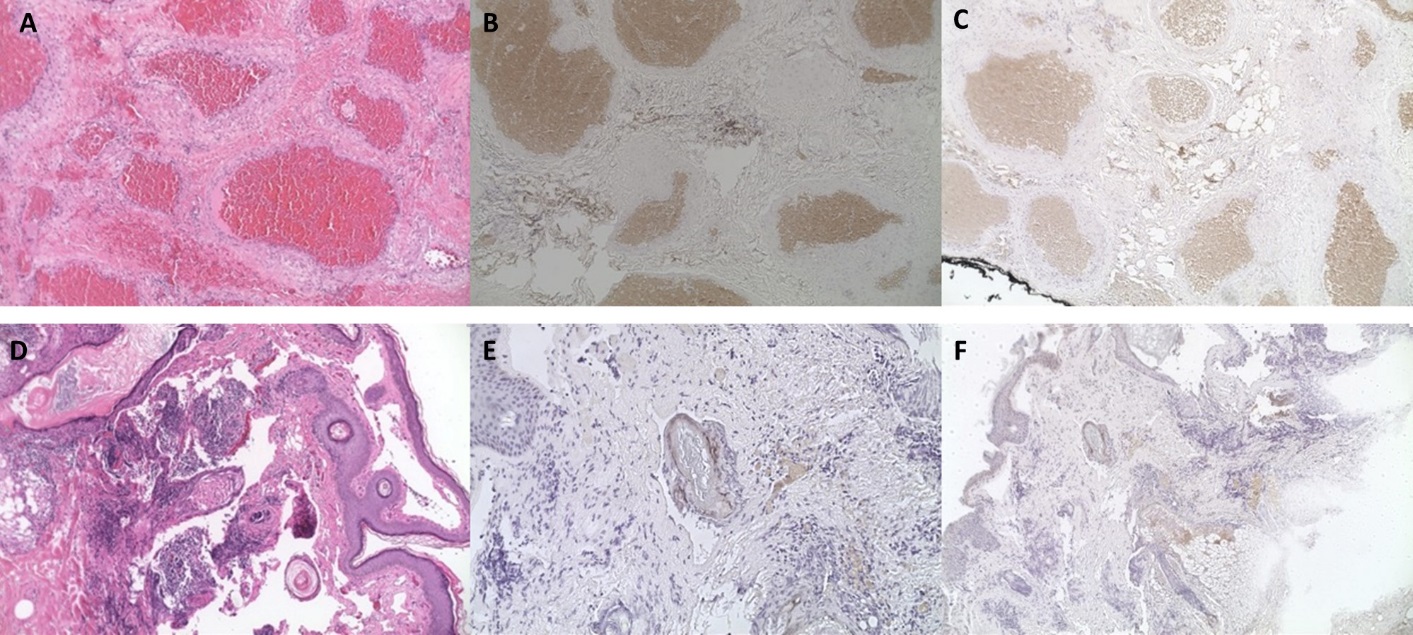
Cavernous malformation hematoxylin and eosin (H&E) immunohistochemical staining at 10x (A) with positivity in VEGFR1 (B) and VEGFR2 (C). Lymphatic malformation hematoxylin and eosin (H&E) immunohistochemical staining lymphangioma (D) with positivity in VEGFR1 (E) and VEGFR2 (F).
Discussion:
Due to their involvement in vascular and lymphatic development and function, VEGF and VEGFRs may play a key role in the pathogenesis and progression of orbital cavernous and lymphatic malformations. In our study, the majority of cavernous malformations demonstrated positive staining for VEGFR1/VEGFR2. However, our lymphatic malformations had lower rates of VEGFR1 and 2 expressions.
There are five structurally related mammalian VEGF ligands (VEGFA, VEGFB, VEGFC, VEGFD, and placenta growth factor PlGF), each of which has variants based on alternative splicing or processing (Tugues et al. 2011). There are three major VEGF receptors: VEGFR1, VEGFR2, and VEGFR3. While not evaluated in our study, VEGFR3 can bind to VEGFC and VEGFD and is involved in lymphatic endothelial cell function and lymphatic development (Tugues et al. 2011).
In our study, the majority of cavernous malformations demonstrated positive staining for VEGFR2. The VEGF pathway may have a pathophysiologic role in development and growth of cavernous malformations, and thus may be a target for therapy. VEGFR2 primarily contributes to angiogenesis through its interactions with VEGFA. VEGFR2 binds to VEGFA, VEGFB, and PlGF. The interaction between VEGFR2 and VEGFA is thought to be a key driver in endothelial cell proliferation and differentiation as well as vascular permeability (Tugues et al. 2011).
Additionally, we found that VEGFR1 was expressed in the majority of cavernous malformations. VEGFR1 is expressed by vascular endothelial and non-endothelial cells. It is induced during vessel growth and remodeling, is required for endothelial cell survival and is upregulated in malignancies (Zhang et al. 2010). VEGFR1 can bind to VEGFA as a negative regulator, serving as a decoy receptor (Weddell et al. 2017). In cavernous malformations, it is possible that VEGFR1 is upregulated in response to increased VEGFR2 and the VEGFA interactions is a method to self-limit growth and rapid expansion. These interactions may be essential for endothelial cell survival even without directly contributing to angiogenesis (Zhang et al. 2010).
Previous studies have investigated immunohistochemical staining of VEGFR in orbital vascular tumors(Table2).
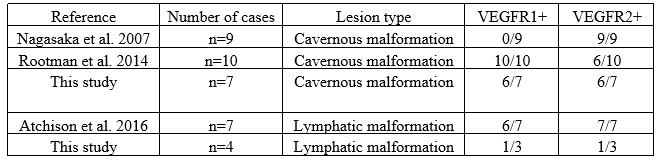
Abbreviations: + = positive, VEGFR1: vascular endothelial growth factor receptor 1, VEGFR2: vascular endothelial growth factor receptor 2
Table 2: Literature Review of VEGFR1 and VEGFR2 Immunohistochemical Staining of Orbital Vascular Tumors.
The first reports on immunohistochemical staining in orbital cavernous malformations found that 0/9 lesions were positive for VEGFR1 but 9/9 stained for VEGFR2 (Nagasaka et al. 2007). Other studies found that 3/11 (27%) of cavernous malformations expressed VEGF but these studies did not evaluate for VEGFR (Gupta et al. 2012). Rootman et al. performed immunohistochemical staining on 10 cavernous malformations. Of these lesions, 10/10 (100%) demonstrated positive staining with VEGFR1 and 6/10 (60%) demonstrated staining with VEGFR2 (Rootman et al. 2014). These results varied from Nagasaka et al. which found higher rates of VEGFR2 than VEGFR1 expression (Nagasaka et al. 2007).
In this study, the prevalence of VEGFR1 and 2 expression in cavernous malformations was similar to prior studies. Atchison et al. was the first to describe VEGFR1 and VEGFR2 expression in lymphatic malformations (Atchison et al. 2016). Their results found higher VEGFR1 and VEGFR2 (6/7 VEGFR1 and 7/7 VEGFR2) compared to our study. This may suggest the possibility of variable VEGFR expression in lymphatic malformations. However, further studies are required to better evaluate the role of VEGFRs and anti-VEGF treatment in lymphatic malformations given the small sample size tested.
Intralesional anti-VEGFA therapy has been used for periocular epithelial hemangiomas as well as intravitreal injections for retinal and choroidal hemangiomas (Mandal et al. 2011, Sagong et al. 2009, Chelala et al. 2013, Kahana et al. 2012). The use of anti-VEGFA for orbital vascular lesions is relatively novel with only limited cases reported. In one case, a cavernous malformation located in the right orbital apex was treated with intralesional bevazicumab (Sweeney et al. 2016). Followed for 2.5 years after the injection, the patient experienced radiographic reduction in lesion size, subjective improvement in dyschromatopsia and complete resolution of visual field defects.
Specifically looking at orbital lymphatic malformations, the combination of bevacizumab and sclerosing agents has been investigated. In one case, the combination of intralesional injections was spaced 6 months apart in a patient with a left orbital lymphatic malformation. Over the next year, the patient experienced improvement in proptosis and regression of the lesion (Mustak et al. 2018). In a case series looking at orbital lymphatic malformations with both macrocystic and microsystic features, a combined injection in the macrocystic components and an injection of bevacizumab into microcytic components resulted reduction in lesion size in two patients (Abdelaziz et al. 2019). These results, however, may be confounded by the simultaneous injection of bleomycin. Other cases of orbital lymphatic malformations have been refractory to intralesional bevacizumab injections and required additional therapy (Gooding et al. 2014).
Limitations of the study include generalizability, small sample size, and staining focused only on VEGFR1 and VEGFR. While newer classifications have been developed to categorize orbital lymphatic malformations, many of our cases did not have imaging available for review, precluding commenting on more venous, lymphatic or arterial flow or the cyst size. Our study also does not investigate the temporal relationship of VEGFR expression and lesion progression.
In summary, this study utilizes immunohistochemical staining to demonstrate that cavernous malformations and lymphatic malformations may express VEGFR1 and VEGFR2. While additional studies are required to further investigate the role of VEGF in these lesions, this study may provide initial insight into the expression of these receptors and support alternative treatment regimens.
Proprietary Interests:
The authors have no conflicts of interest
Financial Support:
Research support from the NIH Center Core Grant P30EY014801
Acknowledgments:
Dr. Nasser Ibrahim Al-Rashid Orbital Vision Research Center. This study was presented at the ASOPRS Fall Annual Meeting 2019, San Francisco, CA