
Hamdi Al-janazreh 1,2*, Reem Alawna 1, Hani Abdeen 1, Shifa Sarahneh 1, Fortunato morabito 2
1Al-Quds University Faculty of Medicine, Jerusalem, State of Palestine
2 Hematology Department and Bone Marrow Transplant Unit, Cancer Care Center, Augusta Victoria Hospital, Jerusalem, State of Palestine
*Corresponding author: Hamdi Al-janazreh, Hematology Department and Bone Marrow Transplant Unit, Cancer Care Center, Augusta Victoria Hospital, Jerusalem, State of Palestine
Received: February 05, 2021
Accepted: February 22, 2021
Published: May 14, 2021
Citation: Hamdi Al-janazreh, Reem Alawna, Hani Abdeen, Shifa Sarahneh, Fortunato morabito, (2021) Blastic Plasmacytoid Dendritic Cell Neoplasm, An Aggressive Malignancy with Unsolved Therapy Questions. A Case Report..J Oncology and Cancer Screening, 2(1); DOI: http;//doi.org/03.2021/1.1003
Copyright: © 2021 Hamdi Al-janazreh, This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
We herein report a case of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare malignancy of myeloid lineage with no well-defined standard therapy. The patient presented the most common skin lesions as well as clear leukemic and lymph node spreading.
Cerebral spinal fluid (CSF) was also clearly involved by malignancy. Tumor cell immunophenotype from skin biopsy (i.e.CD56+, CD45+, CD4+, Tdt+, and CD68+) was consistent with the diagnosis of BPDCN. Bone marrow malignant cells showed common phenotype characteristics. No chromosomal aberration was detected. Since no standard therapy for BPDCN is pondered, and prospective chemotherapy trials are missing, our choice was driven by comprehensive retrospective chemotherapy reviews. Chemotherapy regimens developed for acute myeloid leukemia, partial response. Hopefully, future effort with less unsafe treatments with novel targeted agents will define their place in treating elderly patients.
Introduction
The neoplastic proliferation of plasmacytoid dendritic cells is currently defined as blastic plasmacytoid dendritic cell neoplasm (BPDCN). It is included by the World Health Organization (WHO) classification among the hematological malignancies of myeloid lineage (1). BPDCN most invariable involves the skin, bone marrow, and lymphoid organs and is characterized by CD4 and CD56 positivity associated with the unique expression of myeloid, B- or T- cell lineage markers. This rare disease usually affects the elderly and males most commonly but has been reported in young ages and both sexes (2). Moreover, BPDCN is characterized by numeric and structural genetic aberrations, including RB, IKZF1 acute lymphoid leukemia, and non-Hodgkin lymphoma have been exploited to treat BPDCN. Accordingly, the patient treatment TET2, TP53, NPM1, NRAS, and mLuTta3tions (3). Gene plan consisted of hyper-CVAD, one of the most commonly used first-line treatment regimens in BPDCN patients, followed by allogeneic transplantation, which offers a chance to prolong remission. This judgment was also supported by the disappointing clinical results reached with less intensive therapeutic choice, basically more appropriate for elderly patients. Regrettably, the patient failed to complete the therapeutic program since he died of septic shock after two cycles of arms A and B of the hyper-CVAD protocols after achieving While being highly aggressive with poor
survival outcomes, treatment guidelines are still missing, with no prospective trial available to compare a standardized acute lymphoid leukemia (ALL) versus acute myeloid leukemia (AML) regimens (4).
Case presentation:
Sixty-five-year-old Palestinian male had an asymptomatic mass on the left breast's
growing. At the first clinical evaluation, the bulk was itchy with an abnormal overlying skin characterized by greenish discoloration and a brownish discharge. The patient also had multiple skin lesions ranging from macules to papules with a red to bluish discoloration all over his body. The large mass extended from the anterior axillary line, reaching the chest's midline with severe irregular skin involvement medial to the nipple. In this area, the skin lesion was brownish with yellowish-greenish scale/crustation with irregular borders and shape, surrounded by an erythematous area. The patient also presented diffuse skin nodules worldwide varying in size and color from light pink to bright red to brown. (Figure 1, A and B).
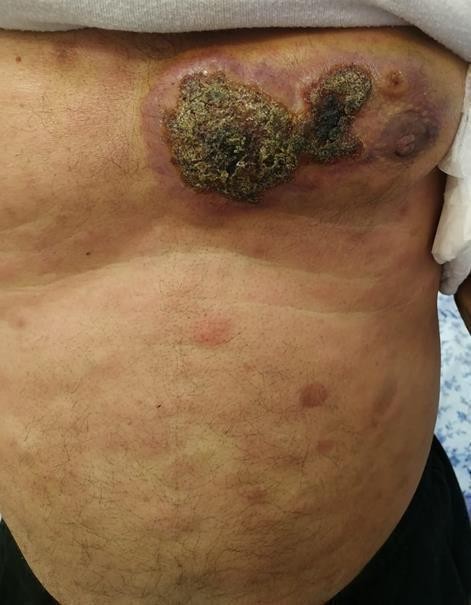
Figure 1A: Extensive skin involvement medial to the left
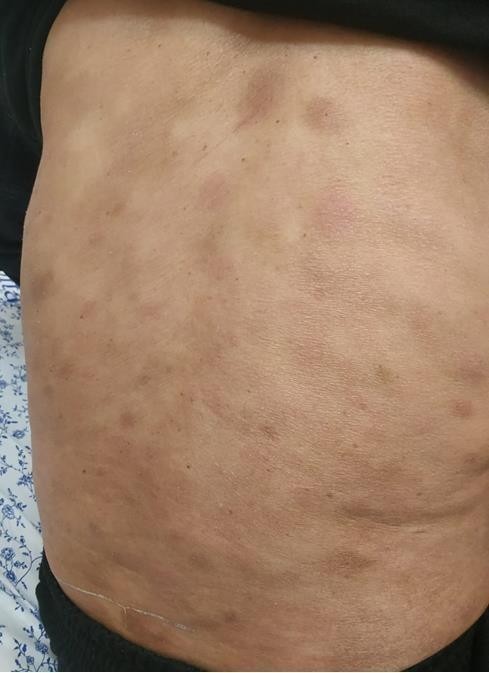
Figure1B: Diffuse skin involvement on the nipple. Diffuse skin lesions all over the abdomen and chest.Back.
(figure1.A) extensive skin involvement medial to the left Figure1.B diffuse skin involvement on the nipple. Diffuse skin lesions all over the abdomen and chest. Back.
The patient’s cell blood count showed mild anemia (Hb 10.3 g/dl), neutropenia (absolute neutrophil count 0.843x10e3/ul), with normal platelet count. The patient had a slightly high Fibrinogen plasma level, PT, PTT, and INR in the normal range. LDH serum level was marginally 396/ul. The blood film showed pancytopenia with the presence of evidence of circulating myoblast. Renal function revealed no abnormal laboratory test, while liver enzymes were ALT 6.6 u/L, AST 21.4 u/L, and ALK:60u/L. All the remaining routine tests were in the normal range.
Neck and abdomen CT showed multiple bilateral cervical, supraclavicular and submental lymph node enlargement.
Furthermore, a chest CT scan showed a large lobulated soft tissue mass measuring 13.5*5.5*2.7cm occupying the left breast area. Multiple surrounding small skin nodules were also detected. Moreover, multiple enlarged lymph nodes were located in both axillae, pretracheal, paratracheal, subcranial, left hilar, and in the posterior aspect of both shoulders. The left lung shows two multiloculated cystic structures without soft component measures 6.2*3.5 cm and 1.4cm. The right lung field was clear with no pleural effusion or thickening.
Abdomen scan also revealed mildly enlarged spleen (long axis 15 cm) with two slit-like peripheral hypodensities, together with multiple enlarged celiac, para-aortic, aortocaval, retro-crural, internal iliac, and bilateral inguinal lymph nodes. Cerebral spinal fluid (CSF) cytology showed numerous large atypical cells consistent with involvement by malignancy.
Karyotyping was normal, with no evidence of clonal, structural, or numerical abnormalities. Skin and breast biopsies showed finding compatible with blastic plasmacytoid dendritic cell neoplasm since tumor cells were positive for CD56, CD45, CD4, Tdt, and CD68, however negative for CD3, CD20, CD30, CD34, C-Kit, myogenin,
desmin. Bone marrow trephine biopsy showed overlapped results. Flow cytometry analysis showed bone marrow blast infiltration of 52%. Blat cells were CD56+ (80%), CD4+ (90%), HLA-DR+(80%). In contrast, lymphoid as well as myeloid markers were negative, suggesting bone marrow involvement of BPDCN (Figure 2)
The planned therapy consisted of chemotherapy followed by AlloBMT. The patient received hyper-CVAD protocol arm A (cyclophosphamide 540 mg on day 1,2,3, doxorubicin (adriamycin) 90mg IV on day 4, vincristine 3.6 mg on day 4, vincristine 2 mg IV on day 11. CNS prophylaxis consisted of methotrexate 12 mg in preserving free saline solution intrathecally on day 2, cytarabine 100mg intrathecally on day 8. G- CSF sc was also administered daily starting from day 5 up to neutrophil recovery.
HyperCVAD arm B (methotrexate 1.8 gm IV over 24 hrs. on day 1, leucovorin and cytarabine 5.4g IV over 2 hours Q 12 hours on day 2, 3; vincristine 2 mg IV on day 4,11; methotrexate 12 mg in free saline solution intrathecally on day2). The patient also received trimethoprim/sulfamethoxazole every other day and acyclovir 400mg po once daily as prophylaxis.
Skin lesions gradually disappeared soon after chemotherapy, and bone marrow cleaned up as well. CT scan documented a pattern of very good partial response.
Due to the COVID-19 pandemic, the patient was admitted to another hospital due to febrile neutropenia and died of septic shock.
Discussion:
BPDCN was initially classified within the acute myeloid leukemia (AML)–related neoplasms in 2008 WHO taxonomy (5) and subsequently relocated as a separate entity in the reviewed version (6). BPDCN typically affects elderly males and is characterized by skin lesions, with or without bone marrow involvement, and leukemic cell spreading in lymphoid organs [7]. The therapeutic approach commonly used for non-Hodgkin lymphoma, acute lymphoblastic leukemia, or AML, i.e. cyclophosphamide, doxorubicin hydrochloride, oncovin, and prednisone (CHOP), hyper-CVAD, and 7+3 (cytarabine, anthracycline) have previously shown to have some clinical efficacy in BPDCN patients (8). Yun et al. showed that among 42 patients who received treatment, the hyper-CVAD regimen was associated with a higher complete response rate (91%) compared with CHOP-based regimens (50%) or SL-401 (50%). However, the difference failed to achieve a statistical significance (9). An international survey on 398 adult patients with BPDCN (10) showed that older age and disseminated disease have an adverse influence on clinical outcome. Conversely, treatment usually utilized in lymphoma and acute leukemia settings followed by transplantation procedures positively impacted survival. Thus, consolidation therapy with either auto or allogeneic bone marrow transplantation is mandatory to improve survival.Several other real-life studies indicate that hyper-CVAD is one of the most commonly used first-line treatment regimens in BPDCN patients, generally showing a remarkable overall response rate (ORR) and a worthy median overall survival (8,11,12,13). Our patient received hyper-CVAD protocol, reaching a partial response. Unfortunately, he died of septic shock as he was unable to complete the therapeutic program. Although we were aware of the decreased chance to meet intensive therapies followed by allo- HSCT consolidation program due to advanced age and comorbidities, we have to underline that in older patients, the results reached with potential more appropriate and less intensive therapeutic choice are disappointing. However, there is a factual basis for the use of novel targeted drugs to treat BPDCN. In this respect, Tagraxofusp (SL-401), an innovative recombinant protein including components of diphtheria toxin merged to interleukin-3, exhibited encouraging results in a phase 1 study (14). More recently, the final results of a phase 2 study evaluating tagraxofusp in 47 patients with BPDCN reported a 90% overall response rate (15). Conclusion:
Intensive therapy followed by consolidation transplantation approaches produced the best effects. The advent of novel agents could be a healthier option for unfit and elderly patients, considering that traditional, less aggressive chemotherapy is still associated with modest outcomes.Nevertheless, prospective trials are unquestionably required to untangle persisting unanswered clinical questions in the treatment of this aggressive malignancy.
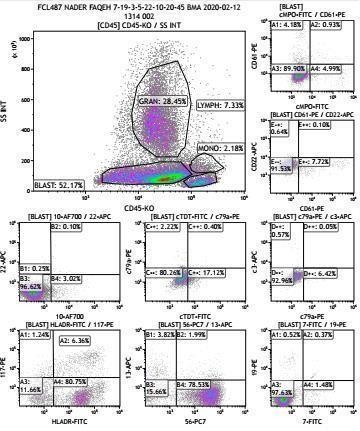
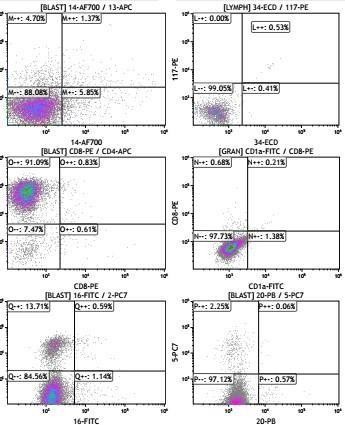
Figure 2: flow cytometry analysis