Bouaita K 1, Benmamar T 1 , Atroune L 2 , Habchi N 2
1Cherchell Hospital, Department of Neurosurgery, Tipaza, Algeria
2Mustapha Bacha Hospital, Department of Neurosurgery, Algiers, Algeria
*Corresponding author: Bouaita Kamel, 1Cherchell Hospital, Department of Neurosurgery, Tipaza, Algeria
Received date: March 05, 2021
Accepted date: March 11, 2021
published date: March 16, 2021
Citation: Bouaita K, Benmamar T, Atroune L, Habchi N. “Anterior Clinoidal Meningioma.”. J Neurosurgery and Neurology Research, 2(2); DOI: http;//doi.org/03.2021/1.1011.
Copyright: © 2021 Bouaita Kamel. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
objective : Anterior clinoidal meningiomas are relatively common benign tumors that are associated with significant morbidity and mortality, primarily from their anatomic proximity to many critical neurological and vascular structures. The purpose of this study is to advocate the standard pterional approach for removing clinoidal meningiomas and to delineate the technique’s advantages that aid in achieving an improved extent of tumor resection and enhacing the patient’s overall outcome, especially their visual outcome.
Methods : A retrospective analysis was performed on 20 consecutive patients with anterior clinoidal meningiomas who underwent surgical resection at the department of Neurosurgery of Cherchell Hospital between January 2015 and June 2020. A standard pterional craniotomy technique was used in all patients. All patients had thorough preoperative and postoperative ophthalmological evaluations. The follow-up period ranged from 6 to 42 months.
Results : Gross total resection was achieved in 13patients (65%) in this series. The majority of the patients with preoperative visual impairment experienced significant visual improvement (60%%). two patients developed permanent complications. The overall recurrence rate was 15%.
Conclusion : in the majority of patients with anterior clinoidal meningioma, gross total resection may be achieved with minimal complications through pterional approach. For large tumors encasing the optic apparatus and large vessels as internal carotid artery or for those tumors causing preoperative visual impairment, the use of the standard pterional craniotomy delineated in this stydy may lead to significant improvement in the patient’s visual and overall outcomes.
Introduction
Sphenoid ridge meningiomas constitute approximately 18% of all intracranial meningiomas. They were classified by Cushing and Eisenhardt into outer (pterional), middle (alar), and inner (deep, clinoidal) lesions according to their site of dural attachment on the lesser sphenoid wing. Anterior clinoidal meningiomas arise from the meningeal covering of the anterior clinoid process. Meningiomas in the anterior clinoid process account for less than half of sphenoid ridge meningiomas. In the past three decades, surgical morbidity and mortality associated with these lesions have been significantly reduced. Nonetheless, even in the microsurgical era, anterior clinoid meningiomas are still reported to have the highest surgical complication and recurrence rate (next to clival meningiomas) among all intracranial meningiomas.
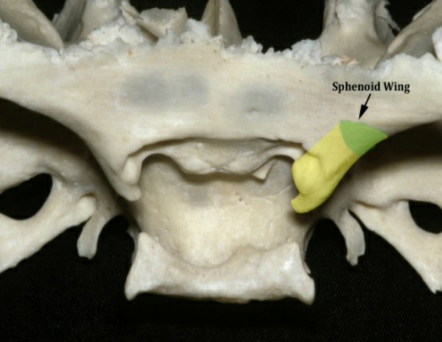
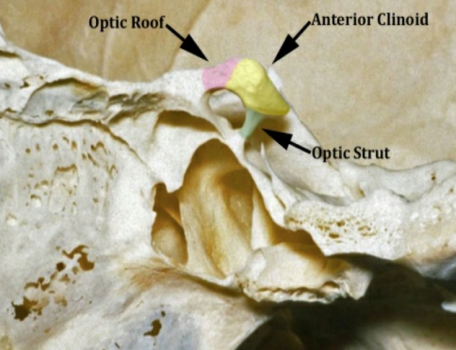
Figure 1: medial sphenoid wing and anterior clinoid process anterior clinoidal meningioma according to Al Mefty’s classification
The purpose of the present study was to evaluate long-term follow-up results in a consecutive series of twenty patients treated microsurgically for an anterior clinoid meningioma.
Visual loss is the very common presenting symptom in these tumors, because of the proximity of the optic nerve to the anterior clinoid process. The characteristic onset usually occurs with unilateral failure of vision loss associated with primary optic atrophy associated with visual field defects in the form of temporal hemianopsia, which is often unrecognized by patients until visual loss is severe and the tumor has reached a significant size.. Vision loss has been reported to occur in 20 to 35% of cases in some series. Headache is another most common symptom in these tumors.
Al Mefty’s classification of clinoidal meningiomas [36,37] has been widely accepted. It differentiates among Group I, lower clinoidal meningiomas (no arachnoidal dissection plane between the internal carotid artery and the tumor); Group II, distal or lateral clinoidal meningiomas (which do have an arachnoidal plane between the ICA and the tumor) and Group III, meningiomas that originate at the optic foramen. In Group III tumors, the arachnoidal membrane is present between the ICA and the tumor but may be absent between the optic nerve and the tumor. Because anterior clinoidal meningiomas tend to grow upward, true invasion of the cavernous sinus is very rare.
Cushing and Eisenhardt first described the surgical resection of sphenoid wing meningiomas in 1938 [4]. Since then, different surgical approaches have been devised to access the sphenoid wing [15,16], such as a frontotemporal/pterional, frontotemporal-orbitozygomatic, and supraorbital-pterional approach, among others. In spite of these developments, as well as advancements in neuroimaging and microsurgical techniques, surgical morbidity and mortality remain unacceptably high. Many surgeons also used intra- or extradural removal of anterior clinoid process for resecting these challenging tumors [6,7,8,9]. The purpose of this study is to advocate the standard pterional approach for removing anterior clinoidal meningiomas and to delineate the technique’s advantages that aid in achieving an improved extent of tumor resection and enhancing the patient’s overall outcome, especially their visual outcome.
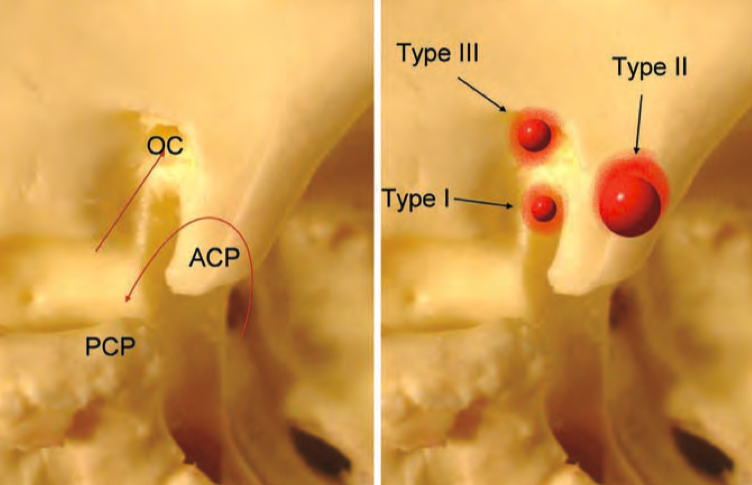
Figure 2: Representative schemes demonstrating differents types of anterior clinoidal meningioma according to Al Mefty’s classification
Materials and Methods
we retrospectively analyzed the clinical records, neuroimaging studies, and long-term follow-up data in a consecutive series of twenty patients harboring anterior clinoid meningiomas. Patients included in this study were treated microsurgically between January 2015 and June 2020 at the department of Neurosurgery of Cherchell Hospital. For patients with anterior clinoid meningioma, we performed rigorous preoperative assessments. All patients underwent preoperative CT scan, multiplanar contrast-enhanced T1-weighted MR images were also obtained. Preoperative CT angiogram was conducted in all patients. Visual testing that included determination of visual acuity, visual field and fundal photography was performed preoperatively and postoperatively in all cases. All patients underwent follow-up with clinical examinations and MR imaging studies six months and one year after surgery. Patients who underwent a radical tumor resection were reevaluated with clinical and radiological assessment at one or two year intervals based on each follow-up result.
Results
1. Patient characteristics : The patients consisted of eight males (40%) and twelve females (60%), with a median age of 56years (range 26-64 years). 10 (50%) of these tumors were detected by minimal symptoms (headache or dizziness) or incidentally. Tumors ranged in size from 1.2 to 8cm (median, 4cm). Intratumoral calcification was documented in four patients (20%). Moderate to severe peritumoral edema formation was evident in six cases (30%). Based on the preoperative CT angiogram and intraoperative confirmation, ICA was encased in five patients (20%).
2. Surgical technique : After induction of general anesthesia, the patient is placed in the supine position, with the head fixed in a Mayfield 3-pin head-holder. The head is then rotated 30 degrees to the side contralateral to the tumor. The head of the bed is elevated approximately 20 degrees. A standard pterional craniotomy is performed. Careful stripping of dura from the bone flap should be conducted. The dura is opened as a flap with caution given to any veins entering the sylvian fissure. Central tumour debulking is performed in a piecemeal fashion once the tumour capsule is entered, it facilitates dissection of the tumor of the surrounding critical neurovascular structures. In firm tumors, an ultrasonic aspirator or careful use of microscissors facilitates piecemeal removal. In most cases, as the arachnoid plane around the optic nerve and internal carotid artery is maintained, careful dissection of the tumor of these critical neurovascular structures is possible. When dissecting the tumor of the optic nerve or the chiasm, fine vessels coursing on the undersurface (which provide the main blood supply) of the optic apparatus must be preserved. In dissecting the tumor extending into the suprasellar region, the pituitary stalk, which is usually displaced posteriorly and medially, must be recognized and preserved. Other neurovascular structures of critical importance include the posterior communicating artery, anterior choroidal artery and their branches, which are encountered during dissection of the inferior pole, and the A1 and M1 main trunks and their branches, which are encountered during dissection/removal of the posterior segment. Once tumor resection is complete, irrigation and haemostasis are essential and strongly recommended.
3. Results: pterional approach was used in the all of the cases. GTR (Simpson’s grade I or II) was possible in thirteen cases (65%), grade III in five cases (25%) and grade IV in two cases (5%). Main causes of interrupting GTR in Simpson’s grade III tumors were the involvement of cavernous sinus (15%) and optic canal (20%). In our series, we found visual improvement in eleven cases (55%), static in seven cases (35%), and deterioration in two case (10%). Papilledema also improved significantly after removal of tumors. The majority of the patients (90%) were diagnosed as a WHO grade I.
4. Complications: In our study most of the patients had limited complications. The most common complication was visual impairment (10%), followed by operation-related hemorrhage or infarction (10%). permanent complications were more prevalent in patient with large tumors (≥4cm), encasement of large vessels and severe peritumoral edema.
5. Follow up : The cranial MRI, visual acuity and visual field were rechecked one to three months after surgery. Thereafter patients were reviewed every six months with MRI to observe the evolution of the lesion. Patients with no residual tumor were followed up every six months. Those with a tumor remnant were scheduled for serial clinical and MR imaging follow-up examinations at yearly intervals. If clinical deterioration or radiological progression of the tumor was evident we proceeded either with reoperation and/or radiotherapy or radiosurgery.
5. Recurrence : Recurrence occurred in two cases (10%) with a median period of 14 months in the non-GTR group (Simpson’s grade III or IV).
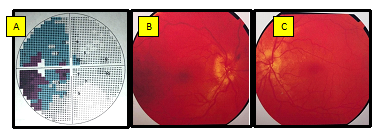
Figure 3: Preoperative visual field test (A) with temporal hemianopsia. Preoperative fundal photography (B right œil, C left eye) with bilateral papilledema.
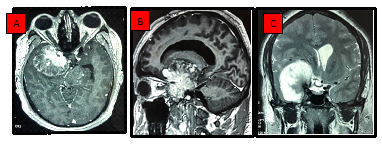
Figure 4: Representative MRI images. Axial and sagittal T1-contrast enhanced weighted MRI (A,B), and coronal T2 weighted MRI (C) showing a huge anterior clinoid meningioma with a consequent mass effect.
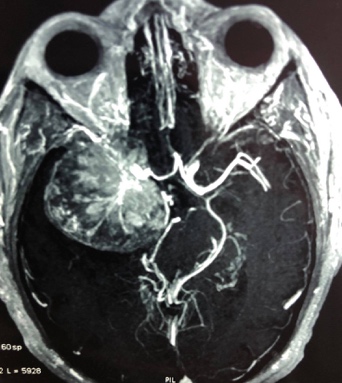
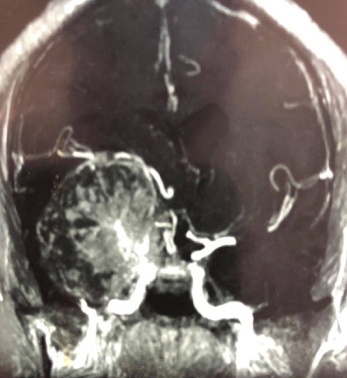
Figure 5: Preoperative CT angiogram showed encasement of large vessels as internal carotid artery and its main branches
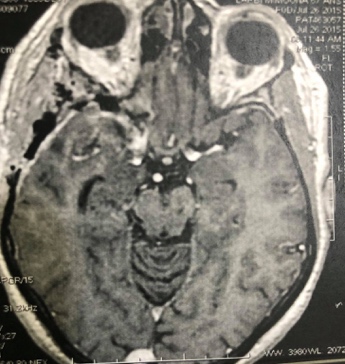
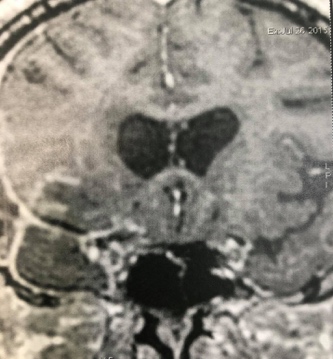
Figure 6: Postoperative axial and coronal T1-contrast enhanced weighted MRI showing gross total resection
Discussion
Meningiomas are relatively common neoplasms that arise from arachnoid cap cells and account for 20% of all primary intracranial tumors [1,2,3]. the incidence of sphenoidal ridge meningiomas ranges from 14 to 23.7% of all intracranial meningiomas, placing them at a complex anatomical location among critical neurological and vascular structures, including the optic nerve, cavernous sinus, and internal carotid and middle cerebral arteries. In 1938, Cushing proposed a classification schemes that divided these tumors into outer or pterional, middle or alar, and deep inner or clinoidal lesions. The clinoidal meningiomas also known in the past as sphenocavernous meningiomas, have the particularity of encasing the internal carotid artery, middle cerebral artery, optic nerve, and often compromises their chance of radical removal and determines very different rates of morbidity, surgical mortality, and recurrences [4]. The grading system used in our study was the one proposed by Al-Mefty in 1990 [5], which takes into consideration not only the point of origin of the meningioma but also its relationship with the internal carotid artery. In fact, Al-Mefty divided clinoidal meningiomas into three groups: Group I was all tumors that have an implant on the lower part of the clinoid and that develop in the carotid cistern and encase the artery, adhering directly to the adventitia in the absence of an arachnoidal membrane; Group II was lesions that originate from the superior or lateral aspect of the anterior clinoid process and that, as they grow, come into contact with the carotid artery, with interposition of an arachnoid membrane deriving from carotid and Sylvian cisterns; Group III was tumors that originate from the optic foramen in which the arachnoid membrane is present between vessels and tumor, but may be lacking between tumor and optic nerve. The presence of the arachnoid membrane is essential for total removal because, by offering a plane of cleavage, it allows the vessels to be completely freed from the tumor. On the whole, it is safe to say that tumors belonging to Groups II and III can be totally removed, in contrast to those of Group I, which often demand subtotal resection to avoid severe postoperative neurological deficits. In the past, total removal of these lesions was not always possible without exposing the patient to a substantial risk of severe neurologic deficits. Nowadays, advances in surgery of the skull, together with the introduction of microsurgical techniques and new diagnostic investigations, have made more radical surgery possible. Patients harboring an anterior clinoid meningioma usually present with unilateral visual complaints [29,30]. In previous series, ophthalmological examination revealed decreased visual acuity in 39 to 92% of patients. The rate of visual field deficits as detected by computed perimetry was 26%. Other neurological symptoms such as seizures, ocular motor nerve palsies, and hemiparesis were also present.
The traditional approach for the resection of anterior clinoidal meningiomas is the pterional intradural transsylvian approach [12,13,14], which begins with splitting the sylvian fissure, releasing cerebrospinal fluid, and debulking the tumor, and then proceeding with peripheral tumor dissection from neurovascular structures. The pterional transsylvian approach has been successfully used by several surgeons for resection of these meningiomas. This approach offers several advantages. It permits a direct approach to the dural attachment of the tumor along the sphenoid ridge and thereby allows for early devascularization. Wide opening of the sylvian fissure improves surgical access and optimizes visualization of all critical neurovascular structures (middle cerebral artery, anterior cerebral artery, internal carotid artery, and optic pathways). Good relaxation of the brain is achieved by opening the basal cisterns and releasing the CSF. The surgical challenges are associated with these giant tumors from their size, difficult location, as well as the dissection, and preservation of the critical neurovascular structures that they inevitably involve or encase. These challenges are increased by tensed brain, secondary edema, and tumor mass effect. However, when cleavage of the arachnoid membrane is not recognizable, dissection of the tumor from the optic nerve and/or chiasm is impossible. In these cases, it seems better to perform only a subtotal removal, considering that recovery of visual deficits after clinoidal meningioma surgery is poor.
Al-Mefty and Ayoubi have reported their results with 28 clinoidal meningiomas [36,37]; they were able to achieve total resection in 83% patients, 71% had a good or fair outcome. Abd El Aziz et al. [47] described a more conservative surgical strategy, where he performed subtotal resection in all of his 17 patients, and 94% of these patients did well postoperatively. Samii and Ammirati, in their analysis of 27 patients, achieved total resection in two thirds, with a good or fair outcome in 77%. Risi et al [34], and Puzilli et al, have reported relatively lower total resection rates (58 and 63%, respectively), with slightly less favorable outcome rate in the 63-64% range.
In the study of Chicani et al. [35] of 65 patients who underwent surgical resection of a medial sphenoid wing meningioma, 46 (71%) maintained their preoperative visual acuity, five (8%) had improved vision, and 14 (22%) had worsened vision at last follow-up. They concluded that male gender, preoperative visual decline, subtotal resection, and need for repeat surgery were all independently associated with postoperative visual decline. Additionally, tumor recurrence and postoperative radiation therapy trended toward an association with visual decline but did achieve statistical significance. The findings of their study support early surgery for patients with medial sphenoid wing meningioma before visual decline occurs and also argues for aggressive surgery to minimize the need for repeat surgery, tumor recurrence, and radiation therapy. Andrews and Wilson [30], in a study of 38 patients with suprasellar meningiomas, documented that tumors involving the optic canal and medial sphenoid wing were most commonly associated with postoperative visual deterioration. Wang et al. [41] found that tumor recurrence, cerebral edema, and preoperative visual decline were associated with visual outcome in 45 patients with suprasellar meningiomas. Nakamura et al. [33] on the other hand, reported that cavernous sinus involvement was associated with poor outcomes after reviewing 55 patients with medial sphenoid wing meningiomas. Al-Mefty et al. [36,37] documented that 24 of 28 patients with clinoidal meningiomas presented with visual disturbances, and only six (25%) improved after surgery. Likewise, Risi et al. [34] found that only 32% of 20 patients with preoperative visual decline had maintained/improved vision following surgical resection. Various studies [21,22,27] demonstrated that the extent of resection was also associated with postoperative visual function. If a tumor was subtotally resected, there was nearly a fourfold increased risk of visual decline at last follow-up. Likewise, need for repeat resection was also independently associated with visual decline at last follow-up [12,13]. Repeat surgery was associated with a near sixfold increased risk of visual decline. Repeat surgery makes it more difficult to achieve safe resection because of increased scarring, loss of arachnoid planes, and a more fibrous tumor. These features emphasize the importance of radical resection at the time of the first surgery to minimize the chance of residual tumor, which has an increased propensity to recur. This recurrence is inherently more difficult to resect. This difficulty may play a role in predisposing patients to visual decline. Interestingly, an association between tumor recurrence and postoperative radiation with decreased visual acuity trended toward significance. These findings further support the importance of radical resection at the first time of surgery to minimize residual tumor, propensity for recurrence, and need for repeat surgery or radiation therapy.
Radiation therapy is a viable adjuvant therapy in treating residual or recurring tumors. In the presence of small clinoidal meningiomas, so long as the tumor is not severely compressing the optic nerve or chiasm, radiosurgery can be performed and a high rate of tumor growth can be arrested (over 90%). The risks to vision are low, but are dependant on the proximity of the optic apparatus [49,50].
Conclusion
Despite the advances in the microsurgical technique and anatomical understanding of the anterior and middle skull base, anterior clinoidal meningiomas are still challenging lesions to resect completely and safely due to their intimate relationship with vital neurovascular structures. in the majority of patients with anterior clinoidal meningioma, gross total resection may be achieved with minimal complications through pterional approach. For large tumors encasing the optic apparatus and large vessels as internal carotid artery or for those tumors causing preoperative visual impairment, the use of the standard pterional craniotomy delineated in this stydy may lead to significant improvement in the patient’s visual and overall outcomes.