Nanda Rachmad Putra Gofur1*, Aisyah Rachmadani Putri Gofur2, Kemal Alif Athallandi2, Soesilaningtyas3, Rizki Nur Rachman Putra Gofur4, Mega Kahdina4, Hernalia Martadila Putri4, 1Department of Health, Faculty of Vocational Studies, Universitas Airlangga, Surabaya, Indonesia
2Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia
3Department of Dental Nursing, Poltekkes Kemenkes, Surabaya, Indonesia
4Faculty Of Medicine, Universitas Airlangga, Surabaya, Indonesia
*Corresponding author: Nanda Rachmad Putra Gofur, Department of Health, Faculty of Vocational Studies, Universitas Airlangga, Surabaya, Indonesia
Received date: April 04, 2021
Accepted date: April 10, 2021
published date: April 19, 2021
Citation: Putra Gofur N R, Putri Gofur A R, Kemal A Athallandi, Soesilaningtyas, Rizki Nur Rachman Putra Gofur, Kahdina M, “Combination of PDLSCs and Nanocomposite-fibrous Scaffold as Alveolar Ridge Preservation in Geriatric Patients before The Prostheses Insertion.”. International Surgery Case Reports, 2(3); DOI: http;//doi.org/03.2021/1.1015.
Copyright: © 2021 Nanda Rachmad Putra Gofur. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Background: In 2050 geriatric sufferers are predicted to be 1.5 billion, representing 16% of the world's population. As many as 50.43% geriatrics experienced tooth loss. In geriatrics, as many as 90% of women will experience a cycle postmenopausal. This condition results in a significant increase in alveolar bone resorption. Decreased body function due to the cycle post menopause can cause a decrease in alveolar ridge which results in denture that is not fit so that the user feels uncomfortable. Therefore, it is necessary to do regenerative treatment for alveolar ridge preservation in geriatrics for prosthesis insertion. Objective: To know the combination of combination of PDLSCs and nanocomposite fibrous scaffold as alveolar ridge preservation in Geriatrics before the insertion of denture prostheses. Discussion: The use of PDLSCs has the ability to differentiate osteogenic by producing growth factors such as BMP-2, VEGF, and FGF-2. BMP-2 plays a role in the stages of the process of osteogenesis and remodeling bone. FGF-2 has a function in cell migration and VEGF plays a role in angiogenesis in areas of bone defects. Nanocomposite fibrous scaffold has a capability dual release system, where there is a combination of release of growth factors produced by PDLSCs (VEGF / BMP-2 and FGF-2 / BMP2) so as to increase bone regeneration and angiogenesis capabilities compared to only a single release system. Conclusion: The combination of PDLSCs and nanocomposite fibrous scaffolds has the potential to maintain the alveolar ridge before prosthesis insertion.
Introduction
By 2050 geriatric patients are predicted to be 1.5 billion, representing 16% of the world's population. Indonesia is a country with a fairly large geriatric population, which in 2030 is predicted to reach 40.09 million people. As many as 50.43% of geriatrics experienced tooth loss. This can affect a person's quality of life. Poor quality of life is not only caused by reduced stomatognathic abilities such as chewing disorders, but also has an impact on self-esteem, where as many as 73.1% of geriatrics with a number of teeth below 11 have a negative impact on the quality of daily life. In geriatrics, as many as 90% of women will experience the menopausal cycle. In menopause, there is a decrease in the hormone estrogen. This results in a decrease in the thickness of the trabecular bone, which is then followed by a decrease in cortical bone thickness. When a geriatric patient uses a protheses such as a denture, the denture is not fit or can easily fall off.1
Geriatric women who experience menopause are more likely to develop osteoporosis which results in rapid alveolar ridge resorption. The alveolar ridges resorption rate was faster during the first six months after extraction and the average yield was 0.5-1.0% per year during life. The healed socket height never reached the past coronal level, and the horizontal resorption was greater in the molar region compared to the premolar area. This leads to the need for repeated adjustments to denture.2
Tissue engineering is a technology in the medical field where a cell can differentiate into several other cells. Tissue engineering consists of 3 components, namely stem cells, scaffold, and growth factors. One of the stem cells that can be used is Periodontal Ligament Stem Cells (PDLSCs). PDLSCs were taken from the impacted third molars and teeth extracted for orthodontic purposes. PDLSCs can produce osteogenic markers that play a role in bone formation. In order for the growth factor produced by stem cells to distribute rapidly in the bone defect area, a nanocomposite fibrous scaffold is also used. Research shows that Mesenchymal Stem Cells (MSCs) derived from dental tissue have advantages in differentiation compared to human Bone Marrow Stem Cells (hBMSCs).3 Alireza et al in their research proved that Periodontal Ligament-Derived Stem Cells (PDLDSCs) have the potential to differentiate into osteogenic tissue. Acemannan is a polysaccharide containing β-(1.4)-acetylated polymannose which is extracted from Aloe vera gel. According to previous research, Acemannan has been shown to stimulate bone formation in vivo.4 Chitosan-composite scaffold is a suitable combination for repairing defects in bone because of its easy-to-manufacture and control characteristics and high mechanical strength.
The use of PLDSCs as a source of stem cells will induce the bone regeneration process because it has osteoinduction and immunomodulatory properties. In addition, the addition of acemannan to PLDSCs will accelerate bone regeneration because it can increase Alkali Phosphatase (ALPase) activity and induce RUNX2 which acts as a transcription factor in osteogenesis.5
Nanocomposite fibrous scaffold has components that resemble chemical components and the architectural shape resembles real bone. Nanocomposite fibrous scaffold has the ability to degrade material and release Si ions, which has been shown to increase osteogenic and endothelial cellular responses in segmental bone defects.6
Therefore, the combination of Periodontal Ligament Stem Cells and Nanocomposite fibrous scaffold has the ability as a good alveolar ridge preservation in geriatrics prior to prosthesis placement. Based on the description above, this paper aims to determine the potential for the combination of periodontal ligament stem cells and fibrous scaffold nanocomposite as alveolar ridge preservation in geriatrics prior to prosthesis placement.
Osteoporosis in Geriatric
There is a close relationship between bone damage and individual age. The process of bone remodeling is the process of removing old bone cells and replacing them with new bone tissue. The process of bone formation is carried out by osteoblasts and the process of bone resorption is carried out by osteoclasts so that this process is useful for maintaining balance to maintain bone mass and bone strength. In geriatric age, there is an imbalance where there is an increase in bone resorption and a decrease in bone formation. Age combined with intrinsic or extrinsic factors (fig 1) can accelerate the decrease in bone mass and thus predispose to bone fracture. This condition is often referred to as osteoporosis. Intrinsic factor consists of genetic, cellular, hormonal, vascular component disorders.7
Osteoporosis is a condition in which there is an abnormality in the skeletal bone characterized by a decrease in bone mass and abnormalities in the constituent of bone tissue which can be found with increased bone fragility and the risk of bone fracture.8
This condition is exacerbated in geriatrics, especially women, who experience menopause in the elderly. Based on data from the World Health Organization (WHO), as many as 30% of women over 50 have osteoporosis. Osteoporosis is caused during the menopause phase, there is a deficiency of the hormone estrogen. The hormone estrogen plays a role in inhibiting osteoclast formation and activity, increasing the production of osteoprotegerin (OPG), or TGF-β. The hormone estrogen also plays a role in suppressing the production of cytokines that play a role in bone resorption such as IL-1, IL-6, TNF-α, M-CSF, and prostaglandins. The hormone estrogen has the ability to directly inhibit osteoclast maturation. This results in a decrease in the thickness of the trabecular bone, which is then followed by a decrease in cortical bone thickness.8,9
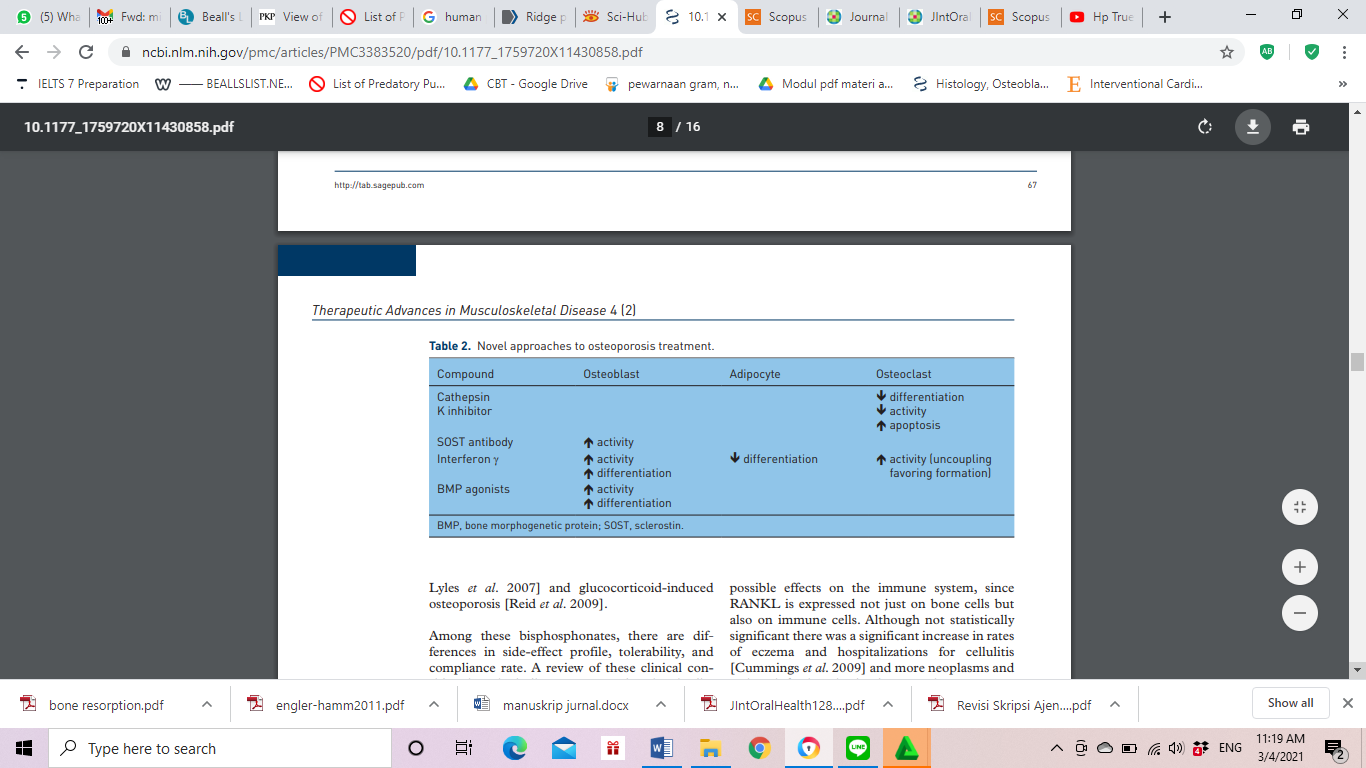
Figure 1: Drug Consumption in Management of Osteoporosis.7
Alveolar Bone Resorption
The maxilla and mandible consist of several anatomical structures with proper function, composition and physiology: (i) the basal bone which develops together with the overall skeleton, and forms the body of the mandible and maxilla; (ii) alveolar processes that develop after tooth eruption and contain dental alveoli; (iii) the bundle of bone that lines the alveolar socket, extends coronally to form the apex of the buccal bone, and forms part of the periodontal structure by encasing the external termination of the periodontal fiber (Sharpey fiber). After tooth extraction, bundle bone is the first bone to be resorbed while alveolar bone is gradually absorbed throughout life. The results of the remodeling process in the morphology of the alveolar ridge are reduced in vertical height and their relationship with natural teeth is more palatal.10
During the first phase, the bone bundle is rapidly absorbed and replaced by woven bone which causes a reduction in bone height, especially in the buccal part because the crest consists only of bundle bone. The buccal plate undergoes more resorption because it is generally thinner, averaging 0.8 mm in the anterior teeth and 1.1 mm in the premolar area.11
The alveolar ridges resorption rate was faster during the first six months after extraction averaging 0.5-1.0% per year during life. The healed socket height never reached the coronal level before, and the horizontal resorption was greater in the molar region compared to the premolar area.2 A recently published systematic review reported greater horizontal alveolar reduction (29-63%; 3.79 mm) than vertical bone loss (11-22%; 1.24 mm in buccal, 0.84 mm mesial, and 0.80 at the distal site) at 6 months.12 In a long-term study, Ashman reported shrinkage of the alveolar bone by 40-60% in height and width in the first 2-3 years.10
Maintenance on Alveolar Ridge
Bone formation in the alveolar socket is an event that occurs naturally as long as the surrounding alveolar wall remains intact; However, volumetric contraction of the alveolar ridge can interfere with implant placement. To reduce alveolar bone resorption, several surgical techniques have been proposed. Reducing extraction trauma and limiting flap elevation are critical to having success in any of these procedures. Animal studies have shown mixed results when evaluating differences in ridge remodeling between flap-used and non-flap extraction sockets.13 It is possible that flap elevation affects changes in alveolar dimensions only in the short term. while in the long term there is no significant difference. In bone regeneration, several methods can be used to increase the rate of bone formation and increase bone volume: osteoinduction using appropriate growth factors; osteoconduction, where the graft material functions as stem cells for new bone growth; distraction osteogenesis, in which a fracture is surgically induced and bone fragments are then slowly pulled apart; thus there is controlled tissue regeneration, which allows space to be filled with new bone.2
Ridge maintenance is indicated in situations where immediate implant placement is not possible for specific patient or location reasons.14 In this scenario, the main meaning of ridge maintenance lies in limiting the contraction of the alveolar ridge during the healing period. A study by Avila-Ortiz et al in 2014 reports effective maintenance of alveolar ridges with a clinical size of 1.89 mm at bucolingual, and 2.07 mm for midbucal height. Maintaining adequate ridge dimensions can help achieve prosthetically driven implant therapy, while avoiding or minimizing the need for additional ridge augmentation at the time of implant placement.1
Teeth Preservation
Tooth extraction is a traumatic procedure that causes the severity of soft tissue and the attachment of the periodontal ligament, along with the disruption of the structure of the blood vessels within the wall of the socket. Therefore, the critical first step is the use of minimally invasive techniques and instruments to prevent additional trauma during extraction (particularly fracture and expansion of the socket wall). After extraction, ridge maintenance techniques can be divided into surgical aspects and material selection aspects.13
1. Surgical Considerations
The flapless approach generally means no soft tissue manipulation or only limited damage is done to allow the expansion of the barrier membrane under the tissue beyond the damaged, and no primary closure is used. In comparison, the use of a flap usually involves a vertical incision, flap reflection and coronal advancement to achieve primary closure.15 Studies report mixed results when comparing the flap and flapless approaches to maintenance of alveolar ridges; some authors describe more pronounced ridge resorption when flap reflection is performed to achieve primary closure.16 While others noted there was no significant difference between the two approaches (Fig 2).17
2. Material Selection
The alveolar ridge maintenance procedure has been tested extensively with various materials and material combinations, such as:
a. Bone grafting only (including autografts, allografts, xenografts and alloplasts)
b. Membrane only (including resorbable or nonresorbable)
c. Combination of membrane and bone graft
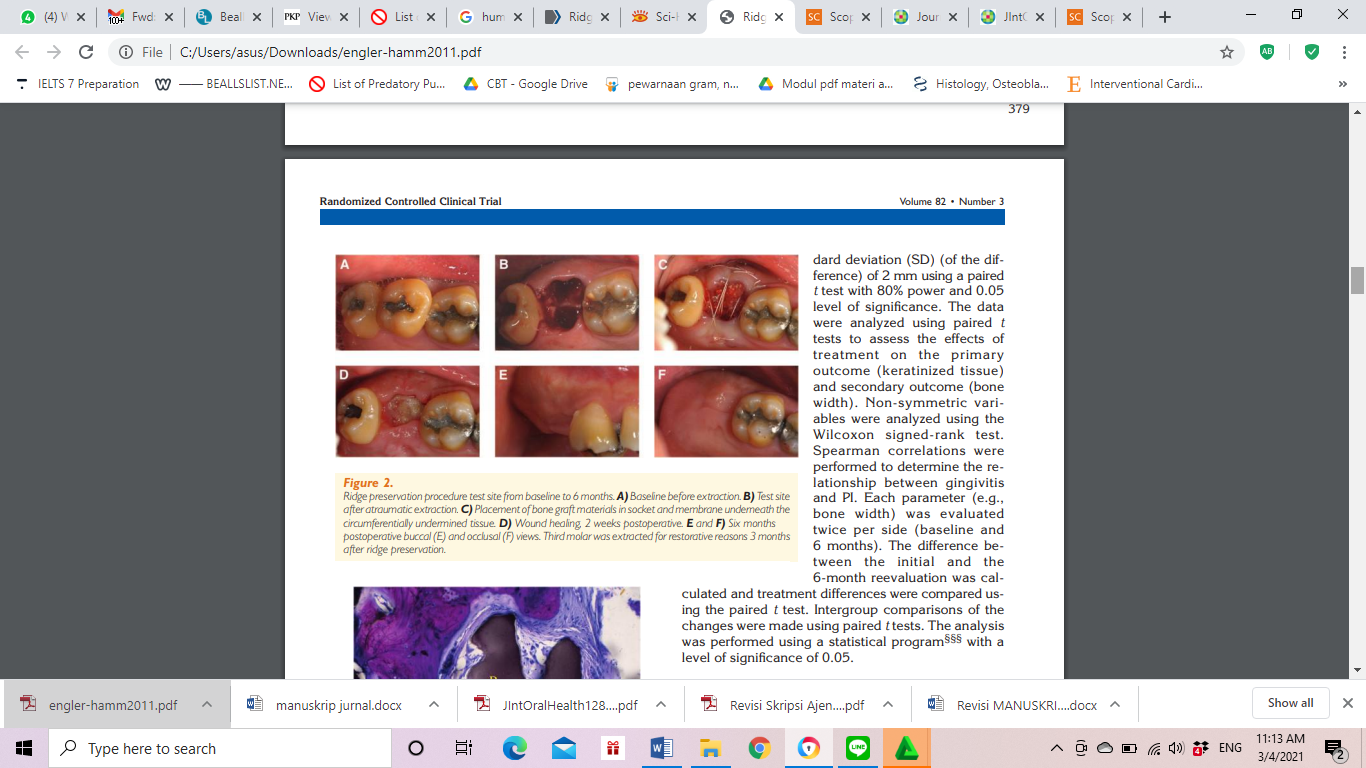
Figure 2: Ridge preservation after the extraction.17
Periodontal Ligament Stem Cells
Periodontal Ligament Stem Cells (PDLSCs) are periodontal ligament stem cells that are part of MSCs (Mesenchymal Stem Cells) and are obtained from taking the entire periodontal tissue from the roots of permanent teeth extracted from the impact process or orthodontic treatment.18 PDLSCs can differentiate into fibroblasts, cementoblasts, and osteoblasts in certain culture media. These cells show alkaline phosphatase (ALP) activity, mineral production, and bone matrix protein for bone regeneration.19
PDLSCs culture processing used enzyme digestion techniques, namely collagenase type I and dispase. This process has a greater proliferation rate, good colony formation efficiency, and a strong differentiation capacity than the use of trypsin and EDTA.20 PDLSCs were cultured in α-MEM (α-Minimum Essential Medium) rather than DMEM (Dulbecco's Minimum Essential Medium) because there are more amino acids, vitamins, and nucleotides so that the proliferation rate is large and the osteogenic potential is stronger. Cells are usually cultured in vitro with a hypoxic state (2% oxygen content) for multipotential maintenance in PDLSCs.20,21 Primary culture will produce small cell sheets for periodontal regeneration according to the protein marker expression in bone regeneration (Fig 3). PDLSCs are highly proliferative and replicative with low density.3,20,22
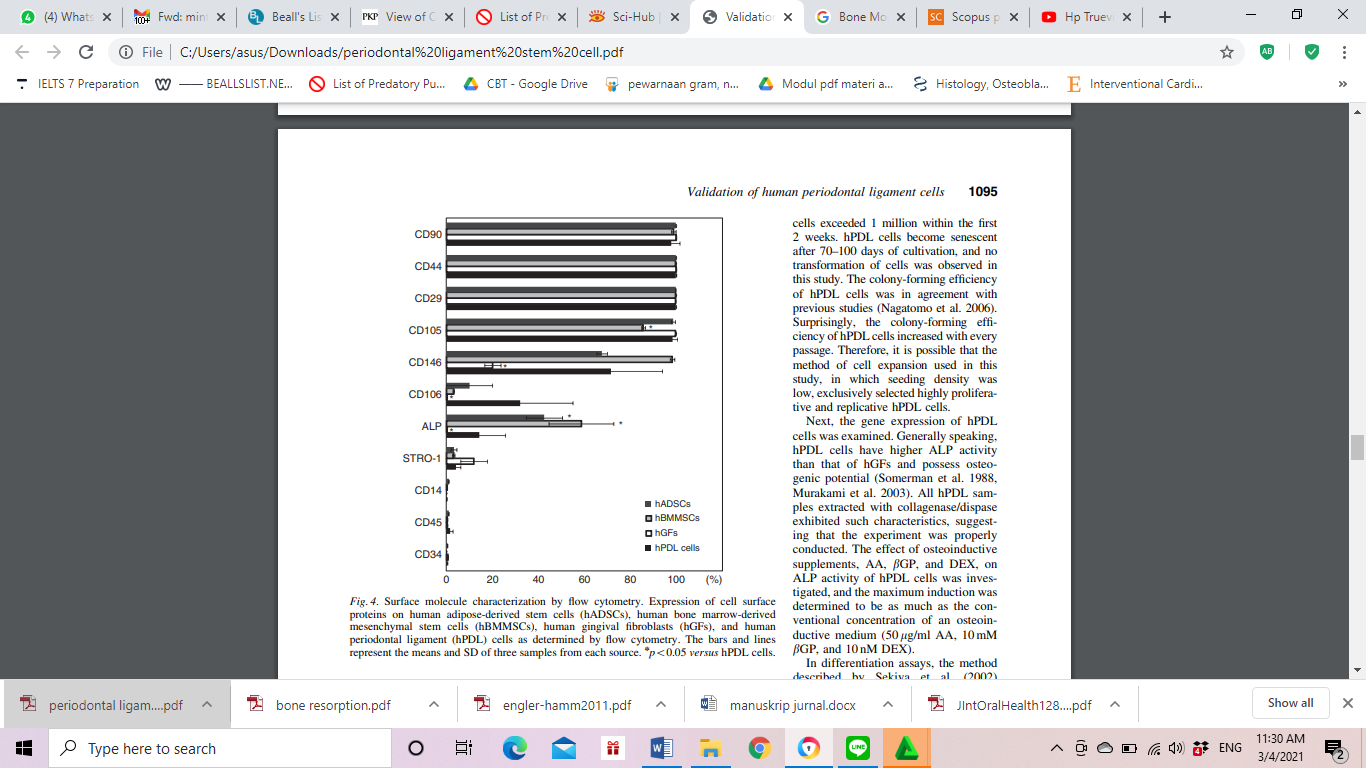
Figure 3: The protein markers expression using flowcytometry in each group of tissue engineering.3
PDLSCs can be used as stem cells because they produce markers for MSCs, such as CD105, CD90, CD73, Stro-1, CD146, and CD44, but they do not have the expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-II.20,23 PDLSCs also have immunomodulatory abilities comparable to bone marrow MSCs.24 This shows that PDLSCs are part of MSCs that are perivascular and almost the same as pericytes.20
PDLSCs express several growth factors such as BMP (Bone Morphogenetic Protein) -2 and -7 and VEGF (Vascular Endothelial Growth Factor) to increase osteogenic differentiation and repair bone damage in animal models.5,25 FGF-2 (Fibroblast Growth Factor-2) can regenerate cementum, alveolar bone, and periodontal ligaments with Sharpey's fibers in class II furcation bone defects in dogs and primates. The use of FGF-2 followed by BMP-2 will increase bone formation activity. The use of FGF-2 for 6 days and followed by BMP-2 for 6 days showed maximum mineralization.20 FGF-2 enhances the osteogenic effect of BMP-2 on PDLSCS by increasing BMP-1B receptor expression and stimulating VEGF secretion.26 In addition, PDLSCs produce several osteogenic markers such as run-related transcription factor-2 (RUNX2), alkaline phosphatase (ALP), and osteocalcin (OCN).27
Nanocomposite-Fibrous Scaffold
Nanocomposite-fibrous scaffold is a local and sustainable scaffold growth factor to form 3D media. This nanocomposite scaffold form has properties such as osteoconductivity, porosity, biodegradability, and mechanical strength that can support cellular and tissue infiltration.6 Fibrous scaffold nanocomposite is formed on nanocomposite scaffold which has a fibrous structure. The main characteristics of these fibrous scaffold nanocomposites are 3D printed to improve mechanical properties and excellent cell compatibility for increased stem cell adhesion, proliferation and differentiation of osteochondral cells in vitro.4
Nanocomposite fibrous scaffold has components that resemble chemical components and the architectural shape resembles real bone. NanoHA coating with amorphous silica coating can increase material degradation and release Si ions, which have been shown to increase osteogenic and endothelial cellular responses in segmental bone defects. Mimicking the chemical composition and fibrous architecture of the original bone, a nano-hydroxyapatite (nanoHA) composite coated with silica-gelatin reinforced with electrospun poly (L-lactic acid) (PLLA) threads has created a fibrous shape on the scaffold.6,28
Discussion
In geriatrics, tooth loss often occurs so it is necessary to replace it in the form of denture because it can interfere with the stomatognathic system such as the mastication system and tooth migration to the edentulous region such as rotation, supraposition, and tipping.29 The use of denture in geriatrics has complications, namely systemic conditions, namely experiencing osteoporosis. This is exacerbated where the elderly women experience the menopause phase. In the menopause phase, estrogen levels decrease. The decrease in the hormone estrogen plays a role in RANKL in increasing osteoclasts and activation and decreasing osteoclast apoptosis. RANKL binds to RANK on osteoclasts and neutralizes the bone microenvironment with OPG. In addition, estrogen also plays a role in suppressing the production of cytokines that play a role in bone resorption such as IL-1, IL-6, TNF-α, M-CSF, and prostaglandins. Estrogen normally increases the production of TGF-β by osteoblasts. TGF-β plays a role in osteoclast apoptosis and decreases osteoclast precursor differentiation by blocking RANKL / M-CSF-induced-activator protein-1-dependent transcription. The decrease in the hormone estrogen results in a decrease in the thickness of the trabecular bone, which is then followed by a decrease in the thickness of the cortical bone. So that it is necessary to reconstruct the alveolar bone in the use of denture by geriatrics, it can be done using tissue engineering which consists of stem cells and scaffold media. One of the stem cells that can be used to regenerate bone is periodontal ligament stem cells that are easily obtained from impacted third molars or an indication of extraction for orthodontic treatment.7,9,30
PDLSCs contain osteoprogenitors which can differentiate into osteoblasts. In addition, PDLSCs inhibit allogenic T cell proliferation through upregulation of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2). PDLSCs suppress proliferation and differentiation of B cells and T cells through cell contact. Low immunogenicity and immunosuppressive effects on T and B cells facilitate allogeneic PDLSCs in tissue regeneration.20,31
PDLSCs have the ability to differentiate osteogenic by generating growth factors such as BMP-2, VEGF, and FGF. BMP-2 plays a role in the stages of the osteogenesis process and bone remodeling. BMP-2 can activate Dlx3 gene transcription via the p38 / Smad5 pathway. This can occur when BMP-2 binds to the BMP-2 receptor, which can activate phosphorylation and translocation of the p38 and Smad5 genes. The phosphorylated p38 gene can also induce phosphorylation of the smad5 gene. This process takes place in the cell membrane. Then this process continues in the nuclear membrane where the phosphorylated p38 gene binds to the phosphorylated Smad5 gene. This process can result in the formation of a protein complex which then binds to the SBEI binding site on the Dlx3 gene promoter. In addition, the Smad5 gene also binds to other proteins to form a protein complex that binds to the SBE II binding site on the Dlx3 gene promoter. The formation of these complex proteins stimulates transcription of the Dlx3 gene to regulate ALP and OC expression. FGF-2 has a function in cell migration, regulating bone density, and the thickness of cortical bone. FGF-2 can play a role in osteogenicity by activating the MAPK pathway and VEGF plays a role in angiogenesis in bone defect areas. VEGF can play a role in angiogenesis by forming new blood vessels so that in the intramembranous ossification process by mesenchymal cells, VEGF can induce the formation of osteoblasts so that new bone tissue can be formed FGF and VEGF also function as chemoatractants for Mesenchymal Stem Cells (MSC) to areas of bone regeneration.5,20,25
The application of PDLSCs in combination with Acemannan requires scaffold so that the stem cells do not undergo denaturation. Nanocomposite fibrous scaffold can be used because it has the ability to accelerate PDLSCs to regenerate bone defects. Nanocomposite fibrous scaffold contains nanoHA which can be the material of choice to support the adhesion and proliferation of osteoblasts and the formation of mineralized bone matrix in vitro. In addition, studies have shown that chitosan is osteoconductive in vivo in surgery causing bone defects. Nanocomposite fibrous scaffold also has the ability to dual release system, where there is a combination of growth factor release produced by PDLSCs (VEGF / BMP-2 and FGF-2 / BMP2) so that it can increase the ability of bone regeneration and angiogenesis compared to just a single release system.28
Other studies have shown that the fibrous scaffold nanocomposite mixture works effectively on bone regeneration. Some indicators such as cell viability and mechanical strength are excellent. Nanocomposite fibrous scaffold will undergo osteogenic differentiation which is played by gene expression from Runx2, ALP, Col, and OCN, and ALP activity. Runx2 induces osteogenic differentiation at an early stage, and inhibits it at a late stage. ALP is essential in the mineralization of ECM with its expression usually indicative of the development of osteogenic differentiation. Col1 and OCN are constituents of ECM and their expression is used as intermediate and late markers of osteogenic differentiation. ALP activity indicates that fibrous scaffold nanocomposites are osteoinductive.4,31
Conclusion
The combination of PDLSCs and fibrous scaffold nanocomposite is a potential alveolar ridge preservation in geriatrics before prothesis.
Conflict of Interest
The authors have declared the is no conflict of interest.